Abstract
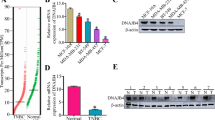
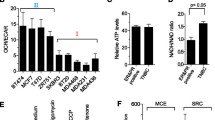
LDH-A, as the critical enzyme accounting for the transformation from pyruvate into lactate, has been demonstrated to be highly expressed in various cancer cells and its silencing has also been approved relating to increased apoptosis in lymphoma cells. In this study, we intend to investigate the correlation between LDH-A and other clinicopathological factors of breast cancer and whether LDH-A silencing could suppress breast cancer growth, and if so the potential mechanisms. 46 breast cancer specimens were collected to study the relation between LDH-A expression and clinicopathological characteristics including menopause, tumor size, node involvement, differentiation, and pathological subtypes classified by ER, PR, and Her-2. shRNAs were designed and applied to silence LDH-A expression in breast cancer cell lines MCF-7 and MDA-MB-231. The effects of LDH-A reduction on cancer cells were studied by a series of in vitro and in vivo experiments, including cell growth assay, apoptosis evaluation, oxidative stress detection, transmission electron microscopy observation, and tumor formation assay on nude mice. LDH-A expression was found to correlate significantly with tumor size and to be independent for other clinicopathological factors. LDH-A reduction resulted in an inhibited cancer cell proliferation, elevated intracellular oxidative stress, and induction of mitochondrial pathway apoptosis. Meanwhile, the tumorigenic ability of LDH-A deficient cancer cells was significantly limited in both breast cancer xenografts. The Ki67 positive cancer cells were significantly reduced in LDH-A deficiency tumor samples, while the apoptosis ratio was enhanced. Our results suggested that LDH-A inhibition might offer a promising therapeutic strategy for breast cancer.





Similar content being viewed by others
Abbreviations
- LDH-A:
-
Lactate dehydrogenase A
- ATP:
-
Adenasine triphosphate
- Her-2:
-
Human epidermal growth factor receptor 2
- G6PD:
-
Glucose-6-phosphate dehydrogenase
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- TKTL1:
-
Transketolase-like protein 1
- NADH:
-
Nicotinamide adenine dinucleotide reduced disodium salt
- TCA:
-
Tricarboxylic acid
- ER:
-
Estrogen receptor
- PR:
-
Progesterone receptor
- shRNA:
-
Short hairpin RNA
- ROS:
-
Reactive oxygen species
- NAC:
-
N-acetylcysteine
- OXPHOS:
-
Oxidative phosphorylation
References
Kitamura T, Taketo MM (2007) Keeping out the bad guys: gateway to cellular target therapy. Cancer Res 67:10099–11002
Stukov AN, Gershanovich ML, Filov VA, Imianitov EN (2005) Problems in the current target therapy of malignancies. Vopr Onkol 51:607–611
Warburg O (1956) On the origin of cancer cell. Science 123:309–314
Warburg O (1956) On respiratory impairment in cancer cell. Science 124:269–270
Shanmugam M, McBrayer SK, Rosen ST (2009) Targeting the Warburg effect in hematological malignancies: from PET to therapy. Curr Opin Oncol 21:531–536
Pelicano H, Martin DS, Xu RH, Huang P (2006) Glycolysis inhibition for anticancer treatment. Oncogene 25:4633–4646
Katabi MM, Chan HL, Karp SE, Batist G (1999) Hexokinase type II: a novel tumor-specific promoter for gene-targeted therapy differentially expressed and regulated in human cancer cells. Hum Gene Ther 10:155–164
Peng Q, Zhou Q, Zhou J, Zhong D, Pan F, Liang H (2008) Stable RNA interference of hexokinase II gene inhibits human colon cancer LoVo cell growth in vitro and in vivo. Cancer Biol Ther 7:1128–1135
Ganapathy-Kanniappan S, Vali M, Kunjithapatham R, Buijs M, Syed LH, Rao PP, Ota S, Kwak BK, Loffroy R, Geschwind JF (2010) 3-bromopyruvate: a new targeted antiglycolytic agent and a promise for cancer therapy. Curr Pharm Biotechnol 11:510–517
Zhu Z, Jiang W, McGinley JN, Thompson HJ (2005) 2-Deoxyglucose as an energy restriction mimetic agent: effects on mammary carcinogenesis and on mammary tumor cell growth in vitro. Cancer Res 65:7023–7030
Stein M, Lin H, Jeyamohan C, Dvorzhinski D, Gounder M, Bray K, Eddy S, Goodin S, White E, Dipaola RS (2010) Targeting tumor metabolism with 2-deoxyglucose in patients with castrate-resistant prostate cancer and advanced malignancies. Prostate 70:1388–1394
Sattler UG, Hirschhaeusera F, Mueller-Klieser WF (2010) Manipulation of glycolysis in malignant tumors: fantasy or therapy? Curr Med Chem 17:96–108
Ganapathy V, Thangaraju M, Prasad PD (2009) Nutrient transporters in cancer: relevance to Warburg hypothesis and beyond. Pharmacol Ther 121:29–40
Granchi C, Bertini S, Macchia M, Minutolo F (2010) Inhibitors of lactate dehydrogenase isoforms and their therapeutic potentials. Curr Med Chem 17:672–697
Koukourakis MI, Giatromanolaki A, Winter S, Leek R, Sivridis E, Harris AL (2009) Lactate dehydrogenase 5 expression in squamous cell head and neck cancer relates to prognosis following radical or postoperative radiotherapy. Oncology 77:285–292
Parolini I, Federici C, Raggi C, Lugini L, Palleschi S, De Milito A, Coscia C, Lessi E, Logozzi M, Molinari A, Colone M, Tatti M, Sargiacomo M, Fais S (2009) Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem 284:34211–34222
Elias AP, Dias S (2008) Microenvironment changes (in pH) affect VEGF alternative splicing. Cancer Microenviron 1(1):131–139
Takahashi Y, Miyajima H, Kaneko E (1995) Genetic analysis of a family of lactate dehydrogenase A subunit deficiency. Intern Med 34:326–329
Maekawa M, Kanno T, Sudo K (1991) Myoglobinuria due to enzyme abnormalities in glycolytic pathway—especially lactate dehydrogenase M subunit deficiency. Rinsho Byori 39:124–132
Le A, Cooper CR, Gouw AM, Dinavahi R, Maitra A, Deck LM, Royer RE, Vander Jagt DL, Semenza GL, Dang CV (2010) Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci USA 107:2037–2042
Giatromanolaki A, Koukourakis MI, Simopoulos C, Polychronidis A, Gatter KC, Harris AL, Sivridis E (2004) c-erbB-2 related aggressiveness in breast cancer is hypoxia inducible factor-1alpha dependent. Clin Cancer Res 10:7972–7977
O’Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, Lane H, Hofmann F, Hicklin DJ, Ludwig DL, Baselga J, Rosen N (2006) mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res 66:1500–1508
Semenza GL (2008) Tumor metabolism: cancer cells give and take lactate. J Clin Invest 118:3835–3857
Robey IF, Stephen RM, Brown KS, Baggett BK, Gatenby RA, Gillies RJ (2008) Regulation of the Warburg effect in early-passage breast cancer cells. Neoplasia 10:745–756
Gründker C, Föst C, Fister S, Nolte N, Günthert AR, Emons G (2010) Gonadotropin-releasing hormone type II antagonist induces apoptosis in MCF-7 and triple-negative MDA-MB-231 human breast cancer cells in vitro and in vivo. Breast Cancer Res 12:R49
Ni Y, Gong XG, Lu M, Chen HM, Wang Y (2008) Mitochondrial ROS burst as an early sign in sarsasapogenin-induced apoptosis in HepG2 cells. Cell Biol Int 32(3):337–343
Indo HP, Davidson M, Yen HC, Suenaga S, Tomita K, Nishii T, Higuchi M, Koga Y, Ozawa T, Majima HJ (2007) Evidence of ROS generation by mitochondria in cells with impaired electron transport chain and mitochondrial DNA damage. Mitochondrion 7(1–2):106–118
Samudio I, Fiegl M, Andreeff M (2009) Mitochondrial uncoupling and the Warburg effect: molecular basis for the reprogramming of cancer cell metabolism. Cancer Res 69:2163–2166
Shen L, Fang H, Chen T, He J, Zhang M, Wei X, Xin Y, Jiang Y, Ding Z, Ji J, Lu J, Bai Y (2010) Evaluating mitochondrial DNA in cancer occurrence and development. Ann NY Acad Sci 1201:26–33
Kulawiec M, Owens KM, Singh KK (2009) Cancer cell mitochondria confer apoptosis resistance and promote metastasis. Cancer Biol Ther 8:1378–1385
Ruckenstuhl C, Büttner S, Carmona-Gutierrez D, Eisenberg T, Kroemer G, Sigrist SJ, Fröhlich KU, Madeo F (2009) The Warburg effect suppresses oxidative stress induced apoptosis in a yeast model for cancer. PLoS one 4:e4592
Kim JS, Ahn KJ, Kim JA, Kim HM, Lee JD, Lee JM, Kim SJ, Park JH (2008) Role of reactive oxygen species-mediated mitochondrial dysregulation in 3-bromopyruvate induced cell death in hepatoma cells: ROS-mediated cell death by 3-BrPA. J Bioenerg Biomembr 40:607–618
Danos M, Taylor WA, Hatch GM (2008) Mitochondrial monolysocardiolipin acyltransferase is elevated in the surviving population of H9c2 cardiac myoblast cells exposed to 2-deoxyglucose-induced apoptosis. Biochem Cell Biol 86:11–20
Acknowledgments
We thank Vicki Geall in the Department of Research Service in The University of Hong Kong for editorial assistance. We also appreciate the great help from The Electronic Microscope Unit in The University of Hong Kong. This research was funded by seed fundings from The University of Hong Kong.
Conflict of interest
The authors declare that they have no potential financial competing interests that may in any way gain or lose financially from the publication of this manuscript at present or in the future. Non-financial competing interests are also not involved in the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, ZY., Loo, T.Y., Shen, JG. et al. LDH-A silencing suppresses breast cancer tumorigenicity through induction of oxidative stress mediated mitochondrial pathway apoptosis. Breast Cancer Res Treat 131, 791–800 (2012). https://doi.org/10.1007/s10549-011-1466-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-011-1466-6