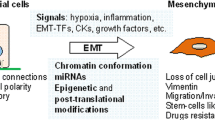
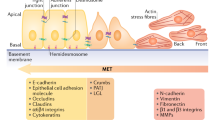
Abstract
Changing the characteristics of cells from epithelial states to mesenchymal properties is a key process involved in developmental and physiological processes as well as in many diseases with cancer as the most prominent example. Nowadays, a great deal of work and literature concerns the understanding of the process of epithelial-to-mesenchymal transition (EMT) in terms of its molecular regulation and its implications for cancer. Similar statements can certainly be made regarding the investigation of the more than 500 proteases typically encoded by a mammalian genome. Specifically, the impact of proteases on tumor biology has been a long-standing topic of interest. However, although EMT actively regulates expression of many proteases and proteolytic enzymes are clearly involved in survival, division, differentiation, and movements of cells, information on the diverse roles of proteases in EMT has been rarely compiled. Here we aim to conceptually connect the scientific areas of “EMT” and “protease” research by describing how several important classes of proteolytic enzymes are regulated by EMT and how they are involved in initiation and execution of the EMT program. To do so, we briefly introduce the evolving key features of EMT and its regulation followed by discussion of protease involvement in this process.

Similar content being viewed by others
Abbreviations
- A549:
-
lung carcinoma cell line
- ADAM:
-
a disintegrin and metalloprotease
- c-MET:
-
MET proto-oncogene
- CRC:
-
colorectal cancer
- CUX1:
-
cut like homeobox 1
- CYLD:
-
CYLD lysine 63 deubiquitinase
- DUB:
-
deubiquitinating enzymes
- e.g. :
-
exempli gratia
- E/M:
-
epithelial/mesenchymal
- ECM:
-
extracellular matrix
- EGFR:
-
epidermal growth factor receptor
- EMT:
-
epithelial-to-mesenchymal transition
- EMT-TF:
-
epithelial-to-mesenchymal transition—transcription factor
- Epcam, Vcam:
-
epithelial cell adhesion molecule, vascular cell adhesion molecule
- ERK:
-
extracellular signal-regulated kinase (also known as mitogen-activated protein kinase 1—MAPK1)
- ESCC:
-
esophageal squamous cell carcinoma
- FGF:
-
fibroblast growth factor
- HCC:
-
hepatocellular carcinoma
- HER:
-
human epidermal growth factor receptor
- HGF:
-
hepatocyte growth factor
- HTRA1:
-
HtrA serine peptidase 1
- i.e. :
-
id est
- IL-6:
-
interleukin-6
- JNK:
-
c-Jun N-terminal kinase
- LA:
-
lung adenocarcinoma
- LGL:
-
lethal giant larvae protein
- LNCaP cells:
-
prostate adenocarcinoma cell from lymph node metastasis
- MCF10A:
-
non-tumorigenic mammary cell line (human origin)
- MCF-7:
-
breast carcinoma cell line
- MET:
-
mesenchymal-to-epithelial transition
- MMP:
-
matrix metalloprotease
- MSPL:
-
mosaic serine protease large-form
- MT-MMP:
-
membrane type matrix metalloprotease
- NMuMG:
-
a non-transformed mouse mammary gland epithelial cell line
- NSCLC:
-
non-small cell lung cancer
- OTUB:
-
OTU deubiquitinase
- PAR:
-
protease-activated receptor
- PATJ:
-
PALS1-associated tight junction protein
- PI3K/Akt/Rac1:
-
phosphatidylinositol-4,5-bisphosphate 3-kinase/AKT serine/threonine kinase 1/Rac1
- Rac1:
-
Ras-related C3 botulinum toxin substrate 1 (rho family, small GTP binding protein Rac1)
- RB:
-
retinoblastoma protein
- ROS:
-
reactive oxygen species
- SENP:
-
sentrin specific protease (family of endopeptidases)
- SMAD:
-
from “MAD—mothers against decapentaplegic” (gene in Drosophila melanogaster) and “Sma—small body size” (protein in Caenorhabditis elegans)
- SW480:
-
human colon cancer cell line
- TGF-β:
-
transforming growth factor-beta
- TMPRSS:
-
transmembrane protease/serine subfamily of the type II transmembrane serine protease (TTSP) family
- TTSP:
-
type II transmembrane serine protease family
- uPA:
-
urokinase-type plasminogen activator
- USP:
-
ubiquitin specific proteases
- Wnt:
-
from “Wg—wingless” (gene in Drosophila melanogaster) and Int-1 (gene in Mus musculus)
- ZEB:
-
zinc finger E-box binding homeobox
- ZO-3:
-
zonula occludens 3, a tight junction protein
References
Greenburg, G., & Hay, E. D. (1982). Epithelia suspended in collagen gels can lose polarity and express characteristics of migrating mesenchymal cells. Journal of Cell Biology, 95(1), 333–339. https://doi.org/10.1083/jcb.95.1.333.
Thiery, J. P., Acloque, H., Huang, R. Y. J., & Nieto, M. A. (2009). Epithelial-mesenchymal transitions in development and disease. Cell, 139(5), 871–890. https://doi.org/10.1016/j.cell.2009.11.007.
Ahmed, N., Maines-Bandiera, S., Quinn, M. A., Unger, W. G., Dedhar, S., & Auersperg, N. (2006). Molecular pathways regulating EGF-induced epithelio-mesenchymal transition in human ovarian surface epithelium. American Journal of Physiology-Cell Physiology, 290(6), C1532–C1542. https://doi.org/10.1152/ajpcell.00478.2005.
Arnoux, V., Nassour, M., L’Helgoualc’h, A., Hipskind, R. A., & Savagner, P. (2008). Erk5 controls slug expression and keratinocyte activation during wound healing. Molecular Biology of the Cell, 19(11), 4738–4749. https://doi.org/10.1091/mbc.e07-10-1078.
Iwano, M., Plieth, D., Danoff, T. M., Xue, C., Okada, H., & Neilson, E. G. (2002). Evidence that fibroblasts derive from epithelium during tissue fibrosis. The Journal of Clinical Investigation, 110(3), 341–350. https://doi.org/10.1172/JCI15518.
Stone, R. C., Pastar, I., Ojeh, N., Chen, V., Liu, S., Garzon, K. I., & Tomic-Canic, M. (2016). Epithelial-mesenchymal transition in tissue repair and fibrosis. Cell and Tissue Research. NIH Public Access. https://doi.org/10.1007/s00441-016-2464-0.
Grande, M. T., Sánchez-Laorden, B., López-Blau, C., De Frutos, C. A., Boutet, A., Arévalo, M., … Nieto, M. A. (2015). Snail1-induced partial epithelial-to-mesenchymal transition drives renal fibrosis in mice and can be targeted to reverse established disease. Nature Medicine, 21(9), 989–997. doi:https://doi.org/10.1038/nm.3901.
Peinado, H., Olmeda, D., & Cano, A. (2007, March). Snail, ZEB and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nature Reviews. Cancer. https://doi.org/10.1038/nrc2131.
Shin, S., Dimitri, C. A., Yoon, S. O., Dowdle, W., & Blenis, J. (2010). ERK2 but not ERK1 induces epithelial-to-mesenchymal transformation via DEF motif-dependent signaling events. Molecular Cell, 38(1), 114–127. https://doi.org/10.1016/j.molcel.2010.02.020.
Janda, E., Lehmann, K., Killisch, I., Jechlinger, M., Herzig, M., Downward, J., … Grünert, S. (2002). Ras and TGFβ cooperatively regulate epithelial cell plasticity and metastasis. The Journal of Cell Biology, 156(2), 299–314. doi:https://doi.org/10.1083/jcb.200109037.
Puisieux, A., Brabletz, T., & Caramel, J. (2014). Oncogenic roles of EMT-inducing transcription factors. Nature Cell Biology. https://doi.org/10.1038/ncb2976.
Stemmler, M. P., Eccles, R. L., Brabletz, S., & Brabletz, T. (2019). Non-redundant functions of EMT transcription factors. Nature Cell Biology. https://doi.org/10.1038/s41556-018-0196-y.
Savagner, P., Yamada, K. M., & Thiery, J. P. (1997). The zinc-finger protein slug causes desmosome dissociation, an initial and necessary step for growth factor–induced epithelial–mesenchymal transition. The Journal of Cell Biology, 137(6), 1403–1419. https://doi.org/10.1083/JCB.137.6.1403.
Vandewalle, C., Comijn, J., De Craene, B., Vermassen, P., Bruyneel, E., Andersen, H., … Berx, G. (2005). SIP1/ZEB2 induces EMT by repressing genes of different epithelial cell-cell junctions. Nucleic Acids Research, 33(20), 6566–78. doi:https://doi.org/10.1093/nar/gki965.
Aigner, K., Dampier, B., Descovich, L., Mikula, M., Sultan, A., Schreiber, M., … Eger, A. (2007). The transcription factor ZEB1 (δEF1) promotes tumour cell dedifferentiation by repressing master regulators of epithelial polarity. Oncogene, 26(49), 6979–6988. doi:https://doi.org/10.1038/sj.onc.1210508.
Spaderna, S., Schmalhofer, O., Wahlbuhl, M., Dimmler, A., Bauer, K., Sultan, A., … Brabletz, T. (2008). The transcriptional repressor ZEB1 promotes metastasis and loss of cell polarity in cancer. Cancer Research, 68(2), 537–544. doi:https://doi.org/10.1158/0008-5472.CAN-07-5682.
Yilmaz, M., & Christofori, G. (2009). EMT, the cytoskeleton, and cancer cell invasion. Cancer and Metastasis Reviews, 28(1–2), 15–33. https://doi.org/10.1007/s10555-008-9169-0.
Miyoshi, A., Kitajima, Y., Sumi, K., Sato, K., Hagiwara, A., Koga, Y., & Miyazaki, K. (2004). Snail and SIP1 increase cancer invasion by upregulating MMP family in hepatocellular carcinoma cells. British Journal of Cancer, 90(6), 1265–1273. https://doi.org/10.1038/sj.bjc.6601685.
Jordà, M., Olmeda, D., Vinyals, A., Valero, E., Cubillo, E., Llorens, A., … Fabra, A. (2005). Upregulation of MMP-9 in MDCK epithelial cell line in response to expression of the snail transcription factor. Journal of Cell Science, 118(Pt 15), 3371–85. doi:https://doi.org/10.1242/jcs.02465.
Hu, F., Wang, C., Guo, S., Sun, W., Mi, D., Gao, Y., … Yang, S. (2011). δEF1 promotes osteolytic metastasis of MDA-MB-231 breast cancer cells by regulating MMP-1 expression. Biochimica et Biophysica Acta (BBA) - Gene Regulatory Mechanisms, 1809(3), 200–210. doi:https://doi.org/10.1016/J.BBAGRM.2011.01.003.
Sanchez-Tillo, E., de Barrios, O., Siles, L., Cuatrecasas, M., Castells, A., & Postigo, A. (2011). Catenin/TCF4 complex induces the epithelial-to-mesenchymal transition (EMT)-activator ZEB1 to regulate tumor invasiveness. Proceedings of the National Academy of Sciences, 108(48), 19204–19209. https://doi.org/10.1073/pnas.1108977108.
Wu, W.-S., You, R.-I., Cheng, C.-C., Lee, M.-C., Lin, T.-Y., & Hu, C.-T. (2017). Snail collaborates with EGR-1 and SP-1 to directly activate transcription of MMP 9 and ZEB1. Scientific Reports, 7(1), 17753. https://doi.org/10.1038/s41598-017-18101-7.
Drak Alsibai, K., & Meseure, D. (2018). Tumor microenvironment and noncoding RNAs as co-drivers of epithelial–mesenchymal transition and cancer metastasis. Developmental Dynamics. John Wiley & Sons, Ltd. doi:https://doi.org/10.1002/dvdy.24548.
Korpal, M., Ell, B. J., Buffa, F. M., Ibrahim, T., Blanco, M. A., Celià-Terrassa, T., … Kang, Y. (2011). Direct targeting of Sec23a by miR-200s influences cancer cell secretome and promotes metastatic colonization. Nature Medicine, 17(9), 1101–1109. doi:https://doi.org/10.1038/nm.2401.
Valastyan, S., & Weinberg, R. A. (2011). Tumor metastasis: molecular insights and evolving paradigms. Cell, 147(2), 275–292. https://doi.org/10.1016/j.cell.2011.09.024.
Brabletz, T. (2012). To differentiate or not—routes towards metastasis. Nature Reviews Cancer, 12(6), 425–436. https://doi.org/10.1038/nrc3265.
Polyak, K., & Weinberg, R. A. (2009, April). Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nature Reviews. Cancer. https://doi.org/10.1038/nrc2620.
Marusyk, A., & Polyak, K. (2010). Tumor heterogeneity: causes and consequences. Biochimica et Biophysica Acta, 1805(1), 105–117. https://doi.org/10.1016/j.bbcan.2009.11.002.
Meacham, C. E., & Morrison, S. J. (2013). Tumor heterogeneity and cancer cell plasticity. Nature, 501(7467), 328. https://doi.org/10.1038/NATURE12624.
Grosse-Wilde, A., Fouquier d’Hérouël, A., McIntosh, E., Ertaylan, G., Skupin, A., Kuestner, R. E., … Huang, S. (2015). Stemness of the hybrid epithelial/mesenchymal state in breast cancer and its association with poor survival. PLoS One, 10(5), e0126522. doi:https://doi.org/10.1371/journal.pone.0126522.
Jolly, M. K., Tripathi, S. C., Jia, D., Mooney, S. M., Celiktas, M., Hanash, S. M., … Levine, H. (2016). Stability of the hybrid epithelial/mesenchymal phenotype. Oncotarget, 7(19), 27067–27084. https://doi.org/10.18632/oncotarget.8166.
Pastushenko, I., Brisebarre, A., Sifrim, A., Fioramonti, M., Revenco, T., Boumahdi, S., … Blanpain, C. (2018). Identification of the tumour transition states occurring during EMT. Nature, 556(7702), 463–468. doi:https://doi.org/10.1038/s41586-018-0040-3.
Kröger, C., Afeyan, A., Mraz, J., Eaton, E. N., Reinhardt, F., Khodor, Y. L., … Weinberg, R. A. (2019). Acquisition of a hybrid E/M state is essential for tumorigenicity of basal breast cancer cells. Proceedings of the National Academy of Sciences, 201812876. doi:https://doi.org/10.1073/pnas.1812876116.
Wiedow, O., & Meyer-Hoffert, U. (2005). Neutrophil serine proteases: Potential key regulators of cell signalling during inflammation. Journal of Internal Medicine. John Wiley & Sons, ltd (10.1111). https://doi.org/10.1111/j.1365-2796.2005.01476.x.
Turk, B., Turk, D., & Turk, V. (2012). Protease signalling: the cutting edge. The EMBO Journal, 31(7), 1630–1643. https://doi.org/10.1038/emboj.2012.42.
Pišlar, A., Perišić Nanut, M., & Kos, J. (2015). Lysosomal cysteine peptidases – molecules signaling tumor cell death and survival. Seminars in Cancer Biology, 35, 168–179. https://doi.org/10.1016/J.SEMCANCER.2015.08.001.
Zhao, P., Metcalf, M., & Bunnett, N. W. (2014). Biased signaling of protease-activated receptors. Frontiers in Endocrinology, 5, 67. https://doi.org/10.3389/fendo.2014.00067.
Winer, A., Adams, S., & Mignatti, P. (2018). Matrix metalloproteinase inhibitors in cancer therapy: turning past failures into future successes. Molecular Cancer Therapeutics, 17(6), 1147–1155. https://doi.org/10.1158/1535-7163.MCT-17-0646.
Coussens, L. M., Fingleton, B., & Matrisian, L. M. (2002). Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science (New York, N.Y.), 295(5564), 2387–2392. https://doi.org/10.1126/science.1067100.
Löser, R., & Pietzsch, J. (2015). Cysteine cathepsins: their role in tumor progression and recent trends in the development of imaging probes. Frontiers in Chemistry, 3, 37. https://doi.org/10.3389/fchem.2015.00037.
Vandooren, J., Opdenakker, G., Loadman, P. M., & Edwards, D. R. (2016). Proteases in cancer drug delivery. Advanced Drug Delivery Reviews, 97, 144–155. https://doi.org/10.1016/j.addr.2015.12.020.
Vidak, E., Javoršek, U., Vizovišek, M., & Turk, B. (2019). Cysteine Cathepsins and their extracellular roles: shaping the microenvironment. Cells, 8(3), 264. https://doi.org/10.3390/cells8030264.
Weidle, U. H., Tiefenthaler, G., & Georges, G. (2014). Proteases as activators for cytotoxic prodrugs in antitumor therapy. Cancer Genomics and Proteomics, 11(2), 67-79.
Sloane, B. F., List, K., Fingleton, B., & Matrisian, L. (2013). Proteases in cancer: significance for invasion and metastasis. In Proteases: structure and function (pp. 491–550). Vienna: Springer Vienna. https://doi.org/10.1007/978-3-7091-0885-7_15.
Sevenich, L., & Joyce, J. A. (2014). Pericellular proteolysis in cancer. Genes & Development, 28(21), 2331–2347. https://doi.org/10.1101/gad.250647.114.
Olson, O. C., & Joyce, J. A. (2015). Cysteine cathepsin proteases: regulators of cancer progression and therapeutic response. Nature Reviews Cancer, 15(12), 712–729. https://doi.org/10.1038/nrc4027.
Mohamed, M. M., & Sloane, B. F. (2006). Multifunctional enzymes in cancer. Nature Reviews Cancer, 6(10), 764–775. https://doi.org/10.1038/nrc1949.
Anja, P., Anahid, J., & Janko, K. (2018). Cysteine cathepsins: their biological and molecular significance in cancer stem cells. Seminars in Cancer Biology, 53, 168–177. https://doi.org/10.1016/J.SEMCANCER.2018.07.010.
Eatemadi, A., Aiyelabegan, H. T., Negahdari, B., Mazlomi, M. A., Daraee, H., Daraee, N., … Sadroddiny, E. (2017). Role of protease and protease inhibitors in cancer pathogenesis and treatment. doi:https://doi.org/10.1016/j.biopha.2016.12.021.
Friedl, P., & Wolf, K. (2010). Plasticity of cell migration: a multiscale tuning model. The Journal of Cell Biology, 188(1), 11–19. https://doi.org/10.1083/jcb.200909003.
Friedl, P., & Alexander, S. (2011). Cancer invasion and the microenvironment: plasticity and reciprocity. Cell Cell Press. https://doi.org/10.1016/j.cell.2011.11.016.
Kessenbrock, K., Plaks, V., & Werb, Z. (2010). Matrix metalloproteinases: regulators of the tumor microenvironment. Cell, 141(1), 52–67. https://doi.org/10.1016/j.cell.2010.03.015.
Duffy, M. J., McKiernan, E., O’Donovan, N., & McGowan, P. M. (2009). Role of ADAMs in cancer formation and progression. Clinical Cancer Research, 15(4), 1140–1144. https://doi.org/10.1158/1078-0432.CCR-08-1585.
Tanabe, L. M., & List, K. (2017). The role of type II transmembrane serine protease-mediated signaling in cancer. The FEBS Journal, 284(10), 1421–1436. https://doi.org/10.1111/febs.13971.
Noë, V., Fingleton, B., Jacobs, K., Crawford, H. C., Vermeulen, S., Steelant, W., … Mareel, M. (2001). Release of an invasion promoter E-cadherin fragment by matrilysin and stromelysin-1. Journal of Cell Science, 114(Pt 1), 111–118.
Deryugina, E. I., Ratnikov, B., Monosov, E., Postnova, T. I., DiScipio, R., Smith, J. W., & Strongin, A. Y. (2001). MT1-MMP initiates activation of pro-MMP-2 and integrin αvβ3 promotes maturation of MMP-2 in breast carcinoma cells. Experimental Cell Research, 263(2), 209–223. https://doi.org/10.1006/EXCR.2000.5118.
Kajita, M., Itoh, Y., Chiba, T., Mori, H., Okada, A., Kinoh, H., & Seiki, M. (2001). Membrane-type 1 matrix metalloproteinase cleaves CD44 and promotes cell migration. Journal of Cell Biology, 153(5), 893–904. https://doi.org/10.1083/jcb.153.5.893.
Symowicz, J., Adley, B. P., Gleason, K. J., Johnson, J. J., Ghosh, S., Fishman, D. A., … Stack, M. S. (2007). Engagement of collagen-binding integrins promotes matrix metalloproteinase-9–dependent E-cadherin ectodomain shedding in ovarian carcinoma cells. Cancer Research, 67(5), 2030–2039. doi:https://doi.org/10.1158/0008-5472.CAN-06-2808.
Lochter, A., Galosy, S., Muschler, J., Freedman, N., Werb, Z., & Bissell, M. J. (1997). Matrix metalloproteinase stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and a premalignant phenotype in mammary epithelial cells. The Journal of Cell Biology, 139(7), 1861–1872.
Maretzky, T., Reiss, K., Ludwig, A., Buchholz, J., Scholz, F., Proksch, E., … Saftig, P. (2005). ADAM10 mediates E-cadherin shedding and regulates epithelial cell-cell adhesion, migration, and beta-catenin translocation. Proceedings of the National Academy of Sciences of the United States of America, 102(26), 9182–7. doi:https://doi.org/10.1073/pnas.0500918102.
Covington, M. D., Burghardt, R. C., & Parrish, A. R. (2006). Ischemia-induced cleavage of cadherins in NRK cells requires MT1-MMP (MMP-14). American Journal of Physiology. Renal Physiology, 290(1), F43–F51. https://doi.org/10.1152/ajprenal.00179.2005.
Egeblad, M., & Werb, Z. (2002). New functions for the matrix metalloproteinases in cancer progression. Nature Reviews Cancer, 2(3), 161–174. https://doi.org/10.1038/nrc745.
Codony-Servat, J., Albanell, J., Lopez-Talavera, J. C., Arribas, J., & Baselga, J. (1999). Cleavage of the HER2 ectodomain is a pervanadate-activable process that is inhibited by the tissue inhibitor of metalloproteases-1 in breast cancer cells. Cancer Research, 59(6), 1196–1201.
Vecchi, M., Rudolph-Owen, L. A., Brown, C. L., Dempsey, P. J., & Carpenter, G. (1998). Tyrosine phosphorylation and proteolysis. Pervanadate-induced, metalloprotease-dependent cleavage of the ErbB-4 receptor and amphiregulin. The Journal of Biological Chemistry, 273(32), 20589–20595. https://doi.org/10.1074/JBC.273.32.20589.
Nath, D., Williamson, N. J., Jarvis, R., & Murphy, G. (2001). Shedding of c-met is regulated by crosstalk between a G-protein coupled receptor and the EGF receptor and is mediated by a TIMP-3 sensitive metalloproteinase. Journal of Cell Science, 114(Pt 6), 1213–1220. https://doi.org/10.1242/jcs.152595.
Illman, S. A., Lehti, K., Keski-Oja, J., & Lohi, J. (2006). Epilysin (MMP-28) induces TGF-beta mediated epithelial to mesenchymal transition in lung carcinoma cells. Journal of Cell Science, 119(Pt 18), 3856–3865. https://doi.org/10.1242/jcs.03157.
Zheng, G., Lyons, J. G., Thian, K. T., Wang, Y., Hsu, T. T., Min, D., … Harris, D. C. H. (2009). Disruption of E-cadherin by matrix metalloproteinase directly mediates epithelial-mesenchymal transition downstream of transforming growth factor-β1 in renal tubular epithelial cells. American Journal of Pathology, 175(2), 580–591. doi:https://doi.org/10.2353/ajpath.2009.080983.
Cheng, S., & Lovett, D. H. (2003). Gelatinase A (MMP-2) is necessary and sufficient for renal tubular cell epithelial-mesenchymal transformation. The American Journal of Pathology, 162(6), 1937–1949. https://doi.org/10.1016/S0002-9440(10)64327-1.
Tan, T. K., Zheng, G., Hsu, T. T., Wang, Y., Lee, V. W. S., Tian, X., … Harris, D. C. H. (2010). Macrophage matrix metalloproteinase-9 mediates epithelial-mesenchymal transition in vitro in murine renal tubular cells. American Journal of Pathology, 176(3), 1256–1270. doi:https://doi.org/10.2353/ajpath.2010.090188.
Cao, J., Chiarelli, C., Richman, O., Zarrabi, K., Kozarekar, P., & Zucker, S. (2008). Membrane type 1 matrix metalloproteinase induces epithelial-to-mesenchymal transition in prostate cancer. The Journal of Biological Chemistry, 283(10), 6232–6240. https://doi.org/10.1074/jbc.M705759200.
Sternlicht, M. D., Bissell, M. J., & Werb, Z. (2000). The matrix metalloproteinase stromelysin-1 acts as a natural mammary tumor promoter. Oncogene, 19(8), 1102–1113. https://doi.org/10.1038/sj.onc.1203347.
Radisky, D. C., Levy, D. D., Littlepage, L. E., Liu, H., Nelson, C. M., Fata, J. E., … Bissell, M. J. (2005). Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature, 436(7047), 123–7. doi:https://doi.org/10.1038/nature03688.
Chen, Q. K., Lee, K., Radisky, D. C., & Nelson, C. M. (2013). Extracellular matrix proteins regulate epithelial-mesenchymal transition in mammary epithelial cells. Differentiation; Research in Biological Diversity, 86(3), 126–132. https://doi.org/10.1016/j.diff.2013.03.003.
Lee, K., Chen, Q. K., Lui, C., Cichon, M. A., Radisky, D. C., & Nelson, C. M. (2012). Matrix compliance regulates Rac1b localization, NADPH oxidase assembly, and epithelial-mesenchymal transition. Molecular Biology of the Cell, 23(20), 4097–4108. https://doi.org/10.1091/mbc.E12-02-0166.
Yu, G., Herazo-Maya, J. D., Nukui, T., Romkes, M., Parwani, A., Juan-Guardela, B. M., … Kass, D. J. (2014). Matrix metalloproteinase-19 promotes metastatic behavior in vitro and is associated with increased mortality in non-small cell lung cancer. American Journal of Respiratory and Critical Care Medicine, 190(7), 780–90. doi:https://doi.org/10.1164/rccm.201310-1903OC.
Pastushenko, I., & Blanpain, C. (2019). EMT transition states during tumor progression and metastasis. Trends in Cell Biology. Elsevier Current Trends. https://doi.org/10.1016/j.tcb.2018.12.001.
Hillebrand, L. E., Wickberg, S. M., Gomez-Auli, A., Follo, M., Maurer, J., Busch, H., … Reinheckel, T. (2018). MMP14 empowers tumor-initiating breast cancer cells under hypoxic nutrient-depleted conditions. The FASEB Journal, 33(3), 4124–4140. doi:https://doi.org/10.1096/fj.201801127r.
Pang, L., Li, Q., Li, S., He, J., Cao, W., Lan, J., … Li, F. (2016). Membrane type 1-matrix metalloproteinase induces epithelial-to-mesenchymal transition in esophageal squamous cell carcinoma: observations from clinical and in vitro analyses. Scientific Reports, 6(1), 22179. doi:https://doi.org/10.1038/srep22179.
Yang, C.-C., Zhu, L.-F., Xu, X.-H., Ning, T.-Y., Ye, J.-H., & Liu, L.-K. (2013). Membrane type 1 matrix metalloproteinase induces an epithelial to mesenchymal transition and cancer stem cell-like properties in SCC9 cells. BMC Cancer, 13(1), 171. https://doi.org/10.1186/1471-2407-13-171.
Nguyen, H.-L., Kadam, P., Helkin, A., Cao, K., Wu, S. J., Samara, G., …, Cao, J. (2016). MT1-MMP activation of TGF-? Signaling enables intercellular activation of an epithelial-mesenchymal transition program in cancer. Current Cancer Drug Targets, 16(7), 618–630. doi:https://doi.org/10.2174/1568009616666160216125634.
Qin, G., Luo, M., Chen, J., Dang, Y., Chen, G., Li, L., …, Yang, J. (2016). Reciprocal activation between MMP-8 and TGF-β1 stimulates EMT and malignant progression of hepatocellular carcinoma. Cancer Letters, 374(1), 85–95. doi:https://doi.org/10.1016/j.canlet.2016.02.001.
Bai, X., Li, Y.-Y., Zhang, H.-Y., Wang, F., He, H.-L., Yao, J.-C., …, Li, S.-S. (2017). Role of matrix metalloproteinase-9 in transforming growth factor-β1-induced epithelial-mesenchymal transition in esophageal squamous cell carcinoma. OncologyTargets and Therapy, 10, 2837–2847. doi:https://doi.org/10.2147/OTT.S134813.
Krstic, J., & Santibanez, J. F. (2014). Transforming growth factor-beta and matrix metalloproteinases: functional interactions in tumor stroma-infiltrating myeloid cells. TheScientificWorldJournal, 2014, 521754. https://doi.org/10.1155/2014/521754.
Santibanez, J. F., Obradović, H., Kukolj, T., & Krstić, J. (2018). Transforming growth factor-β, matrix metalloproteinases, and urokinase-type plasminogen activator interaction in the cancer epithelial to mesenchymal transition. Developmental Dynamics, 247(3), 382–395. https://doi.org/10.1002/dvdy.24554.
Weber, S., & Saftig, P. (2012). Ectodomain shedding and ADAMs in development. Development, 139(20), 3693–3709. https://doi.org/10.1242/DEV.076398.
Murphy, G. (2008). The ADAMs: signalling scissors in the tumour microenvironment. Nature Reviews Cancer, 8(12), 932–941. https://doi.org/10.1038/nrc2459.
Becherer, J. D., & Blobel, C. P. (2003). Biochemical properties and functions of membrane-anchored metalloprotease-disintegrin proteins (ADAMs). Current Topics in Developmental Biology, 54, 101–123. https://doi.org/10.1016/S0070-2153(03)54006-6.
Dong, Y., Wu, Z., He, M., Chen, Y., Chen, Y., Shen, X., … Zeng, Z. (2018). ADAM9 mediates the interleukin-6-induced epithelial–mesenchymal transition and metastasis through ROS production in hepatoma cells. Cancer Letters, 421, 1–14. doi:https://doi.org/10.1016/J.CANLET.2018.02.010.
Pruessmeyer, J., & Ludwig, A. (2009). The good, the bad and the ugly substrates for ADAM10 and ADAM17 in brain pathology, inflammation and cancer. Seminars in Cell & Developmental Biology, 20(2), 164–174. https://doi.org/10.1016/J.SEMCDB.2008.09.005.
Atapattu, L., Saha, N., Chheang, C., Eissman, M. F., Xu, K., Vail, M. E., … Janes, P. W. (2016). An activated form of ADAM10 is tumor selective and regulates cancer stem-like cells and tumor growth. Journal of Experimental Medicine, 213(9), 1741–1757. doi:https://doi.org/10.1084/JEM.20151095.
Cai, M., Wang, Z., Zhang, J., Zhou, H., Jin, L., Bai, R., & Weng, Y. (2015). Adam17, a target of Mir-326, promotes Emt-induced cells invasion in lung adenocarcinoma. Cellular Physiology and Biochemistry, 36(3), 1175–1185. https://doi.org/10.1159/000430288.
Ruff, M., Leyme, A., Le Cann, F., Bonnier, D., Le Seyec, J., Chesnel, F., … Théret, N. (2015). The disintegrin and metalloprotease ADAM12 is associated with TGF-β-induced epithelial to mesenchymal transition. PLoS One, 10(9), e0139179. doi:https://doi.org/10.1371/journal.pone.0139179.
Wang, J., Zhang, Z., Li, R., Mao, F., Sun, W., Chen, J., … Lei, T. (2018). ADAM12 induces EMT and promotes cell migration, invasion and proliferation in pituitary adenomas via EGFR/ERK signaling pathway. Biomedicine & Pharmacotherapy, 97, 1066–1077. doi:https://doi.org/10.1016/J.BIOPHA.2017.11.034.
Najy, A. J., Day, K. C., & Day, M. L. (2008). The ectodomain shedding of E-cadherin by ADAM15 supports ErbB receptor activation. Journal of Biological Chemistry, 283(26), 18393–18401. https://doi.org/10.1074/JBC.M801329200.
Bugge, T. H., Antalis, T. M., & Wu, Q. (2009). Type II transmembrane serine proteases. The Journal of Biological Chemistry, 284(35), 23177–23181. https://doi.org/10.1074/jbc.R109.021006.
Choi, S. Y., Bertram, S., Glowacka, I., Park, Y. W., & Pöhlmann, S. (2009, July 1). Type II transmembrane serine proteases in cancer and viral infections. Trends in Molecular Medicine. Elsevier Current Trends. https://doi.org/10.1016/j.molmed.2009.05.003.
Jung, H., Lee, K. P., Park, S. J., Park, J. H., Jang, Y., Choi, S.-Y., … Park, Y. W. (2008). TMPRSS4 promotes invasion, migration and metastasis of human tumor cells by facilitating an epithelial–mesenchymal transition. Oncogene, 27(18), 2635–2647. doi:https://doi.org/10.1038/sj.onc.1210914.
Wang, C.-H., Guo, Z.-Y., Chen, Z.-T., Zhi, X.-T., Li, D.-K., Dong, Z.-R., … Li, T. (2015). TMPRSS4 facilitates epithelial-mesenchymal transition of hepatocellular carcinoma and is a predictive marker for poor prognosis of patients after curative resection OPEN. doi:https://doi.org/10.1038/srep12366.
Li, S.-L., Chen, X., Wu, T., Zhang, X.-W., Li, H., Zhang, Y., & Ji, Z.-Z. (2018). Knockdown of TMPRSS3 inhibits gastric cancer cell proliferation, invasion and EMT via regulation of the ERK1/2 and PI3K/Akt pathways. Biomedicine & Pharmacotherapy, 107, 841–848. https://doi.org/10.1016/J.BIOPHA.2018.08.023.
Lucas, J. M., Heinlein, C., Kim, T., Hernandez, S. A., Malik, M. S., True, L. D., … Nelson, P. S. (2014). The androgen-regulated protease TMPRSS2 activates aProteolytic cascade involving components of the tumor microenvironment and promotes prostate cancer metastasis. Cancer Discovery, 4(11), 1310. doi:https://doi.org/10.1158/2159-8290.CD-13-1010.
Pawar, N. R., Buzza, M. S., & Antalis, T. M. (2019). Membrane-anchored serine proteases and protease-activated receptor-2–mediated signaling: co-conspirators in cancer progression. Cancer Research, 79(2), 301–310. https://doi.org/10.1158/0008-5472.CAN-18-1745.
Lehner, A., Magdolen, V., Schuster, T., Kotzsch, M., Kiechle, M., Meindl, A., … Gross, E. (2013). Downregulation of serine protease HTRA1 is associated with poor survival in breast cancer. PLoS One, 8(4), e60359. doi:https://doi.org/10.1371/journal.pone.0060359.
Zhu, F., Duan, Y.-F., Bao, W.-Y., Liu, W.-S., Yang, Y., & Cai, H.-H. (2015). HtrA1 regulates epithelial–mesenchymal transition in hepatocellular carcinoma. Biochemical and Biophysical Research Communications, 467(3), 589–594. https://doi.org/10.1016/j.bbrc.2015.09.105.
Wang, N., Eckert, K. A., Zomorrodi, A. R., Xin, P., Pan, W., Shearer, D. A., … Clawson, G. A. (2012). Down-regulation of HtrA1 activates the epithelial-mesenchymal transition and ATM DNA damage response pathways. PLoS One, 7(6), e39446. doi:https://doi.org/10.1371/journal.pone.0039446.
Glickman, M. H., & Ciechanover, A. (2002). The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiological Reviews, 82(2), 373–428. https://doi.org/10.1152/physrev.00027.2001.
Pickart, C. M., & Eddins, M. J. (2004). Ubiquitin: structures, functions, mechanisms. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research, 1695(1–3), 55–72. https://doi.org/10.1016/J.BBAMCR.2004.09.019.
Pfoh, R., Lacdao, I. K., & Saridakis, V. (2015). Deubiquitinases and the new therapeutic opportunities offered to cancer. Endocrine-Related Cancer, 22(1), T35. https://doi.org/10.1530/ERC-14-0516.
Farshi, P., Deshmukh, R. R., Nwankwo, J. O., Arkwright, R. T., Cvek, B., Liu, J., & Dou, Q. P. (2015). Deubiquitinases (DUBs) and DUB inhibitors: a patent review. Expert Opinion on Therapeutic Patents, 25(10), 1191–1208. https://doi.org/10.1517/13543776.2015.1056737.
Yuan, T., Yan, F., Ying, M., Cao, J., He, Q., Zhu, H., & Yang, B. (2018). Inhibition of ubiquitin-specific proteases as a novel anticancer therapeutic strategy. Frontiers in Pharmacology, 9, 1080. https://doi.org/10.3389/fphar.2018.01080.
Choi, B.-J., Park, S.-A., Lee, S.-Y., Cha, Y. N., & Surh, Y.-J. (2017). Hypoxia induces epithelial-mesenchymal transition in colorectal cancer cells through ubiquitin-specific protease 47-mediated stabilization of snail: a potential role of Sox9. Scientific Reports, 7(1), 15918. https://doi.org/10.1038/s41598-017-15139-5.
Meng, J., Ai, X., Lei, Y., Zhong, W., Qian, B., Qiao, K., … Yang, C. (2019). USP5 promotes epithelial-mesenchymal transition by stabilizing SLUG in hepatocellular carcinoma. Theranostics, 9(2), 573–587. doi:https://doi.org/10.7150/thno.27654.
Ouchida, A. T., Kacal, M., Zheng, A., Ambroise, G., Zhang, B., Norberg, E., & Vakifahmetoglu-Norberg, H. (2018). USP10 regulates the stability of the EMT-transcription factor slug/SNAI2. Biochemical and Biophysical Research Communications, 502(4), 429–434. https://doi.org/10.1016/J.BBRC.2018.05.156.
Zeng, Q., Li, Z., Zhao, X., Guo, L., Yu, C., Qin, J., … Yang, X. (2018). Ubiquitin-specific protease 7 promotes osteosarcoma cell metastasis by inducing epithelial-mesenchymal transition. Oncology Reports, 41(1), 543–551. doi:https://doi.org/10.3892/or.2018.6835.
Liu, S., de Boeck, M., van Dam, H., & ten Dijke, P. (2016). Regulation of the TGF-β pathway by deubiquitinases in cancer. The International Journal of Biochemistry & Cell Biology, 76, 135–145. https://doi.org/10.1016/J.BIOCEL.2016.05.001.
Wicks, S. J., Haros, K., Maillard, M., Song, L., Cohen, R. E., ten Dijke, P., & Chantry, A. (2005). The deubiquitinating enzyme UCH37 interacts with Smads and regulates TGF-β signalling. Oncogene, 24(54), 8080–8084. https://doi.org/10.1038/sj.onc.1208944.
Eichhorn, P. J. A., Rodón, L., Gonzàlez-Juncà, A., Dirac, A., Gili, M., Martínez-Sáez, E., et al. (2012). USP15 stabilizes TGF-β receptor I and promotes oncogenesis through the activation of TGF-β signaling in glioblastoma. Nature Medicine, 18(3), 429–435. https://doi.org/10.1038/nm.2619.
Zhang, L., Zhou, F., Drabsch, Y., Gao, R., Snaar-Jagalska, B. E., Mickanin, C., … ten Dijke, P. (2012). USP4 is regulated by AKT phosphorylation and directly deubiquitylates TGF-β type I receptor. Nature Cell Biology, 14(7), 717–726. doi:https://doi.org/10.1038/ncb2522.
Wiener, R., Zhang, X., Wang, T., & Wolberger, C. (2012). The mechanism of OTUB1-mediated inhibition of ubiquitination. Nature, 483(7391), 618–622. https://doi.org/10.1038/nature10911.
Dupont, S., Mamidi, A., Cordenonsi, M., Montagner, M., Zacchigna, L., Adorno, M., … Piccolo, S. (2009). FAM/USP9x, a deubiquitinating enzyme essential for TGFβ signaling, controls Smad4 monoubiquitination. Cell, 136(1), 123–135. doi:https://doi.org/10.1016/J.CELL.2008.10.051.
Zhao, Y., Thornton, A. M., Kinney, M. C., Ma, C. A., Spinner, J. J., Fuss, I. J., … Jain, A. (2011). The deubiquitinase CYLD targets Smad7 protein to regulate transforming growth factor β (TGF-β) signaling and the development of regulatory T cells. The Journal of Biological Chemistry, 286(47), 40520. doi:https://doi.org/10.1074/JBC.M111.292961.
Chanda, A., Sarkar, A., & Bonni, S. (2018). The SUMO system and TGFβ signaling interplay in regulation of epithelial-mesenchymal transition: implications for cancer progression. Cancers, 10(8), E264. https://doi.org/10.3390/cancers10080264.
Chang, C.-C., Huang, Y.-S., Lin, Y.-M., Lin, C.-J., Jeng, J.-C., Liu, S.-M., … Shih, H.-M. (2018). The role of sentrin-specific protease 2 substrate recognition in TGF-β-induced tumorigenesis. Scientific Reports, 8(1), 9786. doi:https://doi.org/10.1038/s41598-018-28103-8.
Chandhoke, A. S., Karve, K., Dadakhujaev, S., Netherton, S., Deng, L., & Bonni, S. (2016). The ubiquitin ligase Smurf2 suppresses TGFβ-induced epithelial–mesenchymal transition in a sumoylation-regulated manner. Cell Death and Differentiation, 23(5), 876. https://doi.org/10.1038/CDD.2015.152.
Stoka, V., Turk, V., & Turk, B. (2016). Lysosomal cathepsins and their regulation in aging and neurodegeneration. Ageing Research Reviews, 32, 22–37. https://doi.org/10.1016/J.ARR.2016.04.010.
Ketterer, S., Gomez-Auli, A., Hillebrand, L. E., Petrera, A., Ketscher, A., & Reinheckel, T. (2017). Inherited diseases caused by mutations in cathepsin protease genes. The FEBS Journal, 284(10), 1437–1454. https://doi.org/10.1111/febs.13980.
van Kasteren, S. I., & Overkleeft, H. S. (2014). Endo-lysosomal proteases in antigen presentation. Current Opinion in Chemical Biology, 23, 8–15. https://doi.org/10.1016/J.CBPA.2014.08.011.
Tan, G.-J., Peng, Z.-K., Lu, J.-P., & Tang, F.-Q. (2013). Cathepsins mediate tumor metastasis. World Journal of Biological Chemistry, 4(4), 91–101. https://doi.org/10.4331/wjbc.v4.i4.91.
Vizovišek, M., Fonović, M., & Turk, B. (2019). Cysteine cathepsins in extracellular matrix remodeling: extracellular matrix degradation and beyond. Matrix Biology, 75–76, 141–159. https://doi.org/10.1016/J.MATBIO.2018.01.024.
Aits, S., & Jä, M. (n.d.). Lysosomal cell death at a glance. Journal of Cell Science, 126, 1905–1912. https://doi.org/10.1242/jcs.091181.
Campden, R. I., & Zhang, Y. (2019). The role of lysosomal cysteine cathepsins in NLRP3 inflammasome activation. Archives of Biochemistry and Biophysics. https://doi.org/10.1016/J.ABB.2019.02.015.
Kern, U., Wischnewski, V., Biniossek, M. L., Schilling, O., & Reinheckel, T. (2015). Lysosomal protein turnover contributes to the acquisition of TGFβ-1 induced invasive properties of mammary cancer cells. Molecular Cancer, 14(1), 39. https://doi.org/10.1186/s12943-015-0313-5.
Kryczka, J., Papiewska-Pajak, I., Kowalska, M. A., Boncela, J., Kryczka, J., Papiewska-Pajak, I., … Boncela, J. (2019). Cathepsin B is upregulated and mediates ECM degradation in colon adenocarcinoma HT29 cells overexpressing Snail. Cells, 8(3), 203. doi:https://doi.org/10.3390/cells8030203.
Mitrović, A., Pečar Fonović, U., & Kos, J. (2017). Cysteine cathepsins B and X promote epithelial-mesenchymal transition of tumor cells. European Journal of Cell Biology, 96(6), 622–631. https://doi.org/10.1016/J.EJCB.2017.04.003.
Wang, J., Chen, L., Li, Y., & Guan, X.-Y. (2011). Overexpression of cathepsin Z contributes to tumor metastasis by inducing epithelial-mesenchymal transition in hepatocellular carcinoma. PLoS One, 6(9), e24967. https://doi.org/10.1371/journal.pone.0024967.
Burton, L. J., Dougan, J., Jones, J., Smith, B. N., Randle, D., Henderson, V., & Odero-Marah, V. A. (2017). Targeting the nuclear Cathepsin L CCAAT displacement protein/cut Homeobox transcription factor-epithelial mesenchymal transition pathway in prostate and breast cancer cells with the Z-FY-CHO inhibitor. Molecular and Cellular Biology, 37(5), e00297–e00216. https://doi.org/10.1128/MCB.00297-16.
Kedinger, V., Sansregret, L., Harada, R., Vadnais, C., Cadieux, C., Fathers, K., … Nepveu, A. (2009). p110 CUX1 homeodomain protein stimulates cell migration and invasion in part through a regulatory cascade culminating in the repression of E-cadherin and occludin. The Journal of Biological Chemistry, 284(40), 27701–11. doi:https://doi.org/10.1074/jbc.M109.031849.
Han, M.-L., Zhao, Y.-F., Tan, C.-H., Xiong, Y.-J., Wang, W.-J., Wu, F., … Liang, Z.-Q. (2016). Cathepsin L upregulation-induced EMT phenotype is associated with the acquisition of cisplatin or paclitaxel resistance in A549 cells. Acta Pharmacologica Sinica, 37(12), 1606–1622. doi:https://doi.org/10.1038/aps.2016.93.
Wang, W., Xiong, Y., Ding, X., Wang, L., Zhao, Y., Fei, Y., … Liang, Z. (2019). Cathepsin L activated by mutant p53 and Egr-1 promotes ionizing radiation-induced EMT in human NSCLC. Journal of Experimental & Clinical Cancer Research, 38(1), 61. doi:https://doi.org/10.1186/s13046-019-1054-x.
Zhang, Q., Han, M., Wang, W., Song, Y., Chen, G., Wang, Z., & Liang, Z. (2015). Downregulation of cathepsin L suppresses cancer invasion and migration by inhibiting transforming growth factor-β-mediated epithelial-mesenchymal transition. Oncology Reports, 33(4), 1851–1859. https://doi.org/10.3892/or.2015.3754.
Bonnans, C., Chou, J., & Werb, Z. (2014). Remodelling the extracellular matrix in development and disease. Nature Reviews. Molecular Cell Biology, 15(12), 786–801. https://doi.org/10.1038/nrm3904.
Conlon, G. A., & Murray, G. I. (2019). Recent advances in understanding the roles of matrix metalloproteinases in tumour invasion and metastasis. The Journal of Pathology, 247(5), 629–640. https://doi.org/10.1002/path.5225.
Overall, C. M., & Dean, R. A. (2006). Degradomics: systems biology of the protease web. Pleiotropic roles of MMPs in cancer. Cancer and Metastasis Reviews, 25(1), 69–75. https://doi.org/10.1007/s10555-006-7890-0.
Lawrence, R. E., & Zoncu, R. (2019). The lysosome as a cellular centre for signalling, metabolism and quality control. Nature Cell Biology, 21(2), 133–142. https://doi.org/10.1038/s41556-018-0244-7.
López-Otín, C., & Matrisian, L. M. (2007). Emerging roles of proteases in tumour suppression. Nature Reviews Cancer, 7(10), 800–808. https://doi.org/10.1038/nrc2228.
Dennemärker, J., Lohmüller, T., Mayerle, J., Tacke, M., Lerch, M. M., Coussens, L. M., … Reinheckel, T. (2010). Deficiency for the cysteine protease cathepsin L promotes tumor progression in mouse epidermis. Oncogene, 29(11), 1611–21. doi:https://doi.org/10.1038/onc.2009.466.
Acknowledgements
The authors thank Eva Schill-Wendt for creating the artwork of the figure. The Deutsche Forschungsgemeinschaft (DFG) SFB 850 subproject B7, DFG-grant RE1584/6-2, and the German Cancer Consortium (DKTK) program Oncogenic Pathways project L627 support the work in the Reinheckel laboratory.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mitschke, J., Burk, U.C. & Reinheckel, T. The role of proteases in epithelial-to-mesenchymal cell transitions in cancer. Cancer Metastasis Rev 38, 431–444 (2019). https://doi.org/10.1007/s10555-019-09808-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-019-09808-2