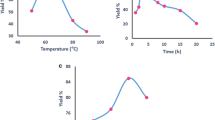
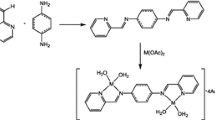
Benzoquinone, diphenoquinones and its derivatives are important intermediates for industrial synthesis of a wide variety of special chemicals, such as pharmaceuticals, dyes and agricultural chemicals. The useful catalyst were obtained by aminolysis of vinylbenzyl chloride/divinylbenzene copolymer with ethylenediamine (1) or urotropine (2) and then modification by salicylaldehyde (1A, 2A) or picolinaldehyde (1B, 2B). The catalytic activity of Cu(II) complexes with Schiff base immobilized on the synthesized supports were tested in the oxidation reaction of 2,6-di-tert-butylphenol (DTBP) to diphenoquinone (PQ) with tert-butylhydroperoxide. The best oxidation degree of DTBP (60-70%) and the selectivity towards PQ (80%) is revealed by Cu(II) complexes with long Schiff base ligands derived from salicylaldimine (1A), which have CuL structure (EPR measurement).
Similar content being viewed by others
References
N.E. Leadbeater M. Marco (2002) Chem. Rev. 102 3248 Occurrence Handle10.1021/cr010361c
D.C. Sherrington (2000) Catal. Today 57 87 Occurrence Handle10.1016/S0920-5861(99)00311-9 Occurrence Handle1:CAS:528:DC%2BD3cXhsFensbs%3D
E.A. Becturov S.E. Kudaibergov (1996) Catalysis by Polymers Zug. Heidenlberg Oxford
A.S. Hay (1999) Prog. Polym. Sci. 24 45 Occurrence Handle10.1016/S0079-6700(98)00016-1 Occurrence Handle1:CAS:528:DyaK1MXktVersr8%3D
P.G. Aubel S.S. Khokhar W.I. Dressen G. Challa J. Reedijk (2001) J. Mol. Catal. A: Chem. 175 27 Occurrence Handle10.1016/S1381-1169(01)00212-6 Occurrence Handle1:CAS:528:DC%2BD3MXnt1Wit7c%3D
P. Gamez C. Simons R. Steensma W.Ł Drissen G. Challa J. Reedijk (2001) Eur. Polym. J. 37 1293 Occurrence Handle1:CAS:528:DC%2BD3MXjslSjtL0%3D
P.C. Selvaraj V. Mahadevan (1998) Polymer 39 1741 Occurrence Handle10.1016/S0032-3861(97)00500-4 Occurrence Handle1:CAS:528:DyaK1cXksFyqtQ%3D%3D
VR. Gupta R. Mukherjee (2000) Tetrahedron Lett. 41 7763 Occurrence Handle1:CAS:528:DC%2BD3cXntF2lsro%3D
I.G. Kolesnik E.G. Zhizhina K.I. Matveev (2000) J. Mol. Catal. A: Chem. 154 147
A. Puzari J.B. Baruah (2002) J. Mol. Catal. A: Chem. 187 140 Occurrence Handle10.1016/S1381-1169(02)00273-X
K. Takemoto R.M. Ottenbrite M. Kamach (1999) Functional Monomers and Polymers Marcel Dekker. Ins New York
D.A. Rockcliffe A.E. Martell (1995) J. Mol. Catal. A: Chem. 99 101 Occurrence Handle1:CAS:528:DyaK2MXmvVGgsL0%3D
D.A. Rockcliffe A.E. Martell (1996) J. Mol. Catal. A: Chem. 106 211 Occurrence Handle10.1016/1381-1169(95)00272-3 Occurrence Handle1:CAS:528:DyaK28XhvVKktLs%3D
R. Path G.N. Rao (1998) J. Mol. Catal. A: Chem. 130 215
H. Fujiyama I. Kohara K. Iwai S. Nishiyama S. Tsruya M. Masai (1999) J. Catal. 188 417 Occurrence Handle10.1006/jcat.1999.2667 Occurrence Handle1:CAS:528:DyaK1MXotFeksbw%3D
I. Kohara H. Fujiyama K. Iwai S. Nishiyama S. Tsuruya (2000) J. Mol. Catal. A: Chem. 153 93 Occurrence Handle10.1016/S1381-1169(99)00306-4 Occurrence Handle1:CAS:528:DC%2BD3cXhs1yqsbk%3D
K. Omura (2000) Tetrahedron Lett. 41 685 Occurrence Handle10.1016/S0040-4039(99)02139-5
A.M. Guidote SuffixJr. K. Ando K. Terada Y. Kurusu H. Nagao Y. Masuyama (2001) Inorg. Chim. Acta 324 203 Occurrence Handle10.1016/S0020-1693(01)00589-8 Occurrence Handle1:CAS:528:DC%2BD3MXosFGhtbk%3D
Y. Kursu (2002) Macromol. Symp. 186 7
K. Takaki Y. Shimasaki T. Shishido K. Takehira (2002) Bull. Chem. Soc. Jpn. 75 111 Occurrence Handle10.1246/bcsj.75.311
A. Pui I. Berdan I. Morgenstern-Badarau A. Gref M. Perree-Fauvet (2001) Inorg. Chim. Acta 320 167 Occurrence Handle10.1016/S0020-1693(01)00463-7 Occurrence Handle1:CAS:528:DC%2BD3MXlslGhuro%3D
C. Comuzzi A. Melchior P. Polese R. Portanova M. Tolazzi (2003) Inorg. Chim. Acta 355 57 Occurrence Handle10.1016/S0020-1693(03)00235-4 Occurrence Handle1:CAS:528:DC%2BD3sXptlOgur4%3D
G.T. Musie M. Weis B. Subramaniam D.H. Busch (2001) Inorg. Chem. 40 3336 Occurrence Handle10.1021/ic001288w Occurrence Handle1:CAS:528:DC%2BD3MXjvFeqt7g%3D
M. Wei G.T. Musie D.H. Busch B. Subramaniam (2004) Green Chem. 6 387 Occurrence Handle10.1039/b310523g Occurrence Handle1:CAS:528:DC%2BD2cXnsVyjtb8%3D
P. Mastrorilli F. Muscio G.P. Suranna C.F. Nobile M. Latronico (2001) J. Mol. Catal. A: Chem. 165 81 Occurrence Handle10.1016/S1381-1169(00)00437-4 Occurrence Handle1:CAS:528:DC%2BD3MXhtVOisbw%3D
W.A. Alves I.A. Bagatin A.M.D.C. Ferreira (2001) Inorg. Chim. Acta 321 11 Occurrence Handle10.1016/S0020-1693(01)00490-X Occurrence Handle1:CAS:528:DC%2BD3MXms1Omtb0%3D
W.A. Alves S.A. Almeida-Filho Particlede M.V. Almeida Particlede A. Paduan-Filho C.C. Becerra A.M.D.C. Ferreira (2003) J. Mol. Catal. A: Chem. 198 63 Occurrence Handle10.1016/S1381-1169(03)00003-7 Occurrence Handle1:CAS:528:DC%2BD3sXjt1Kiurs%3D
A. Warshawsky A. Deshe G. Rossey A. Patchornik (1984) React. Polym. 2 301 Occurrence Handle1:CAS:528:DyaL2cXlslWrurg%3D
B.N. Kolarz J. Jezierska D. Bartkowiak A. Gontarczyk (1994) React. Polym. 23 53 Occurrence Handle1:CAS:528:DyaK2cXmvVWnsrY%3D
I. Owsik B.N. Kolarz (2002) J. Mol. Catal. A: Chem. 178 63 Occurrence Handle10.1016/S1381-1169(01)00299-0 Occurrence Handle1:CAS:528:DC%2BD38XmtFyq
B. JeŻowska-Trzebiatowska J. Jegierska (1973) J. Mol. Struct. 19 627
I. Kuźniarka-Biernacka K. Kurzak B. Kurzak J. Jezierska (2003) J. Solut. Chem. 32 719
T. Okuno S. Ohba Y. Nishida (1997) Polyhedron 16 3765 Occurrence Handle10.1016/S0277-5387(97)00147-2 Occurrence Handle1:CAS:528:DyaK2sXlvV2lt78%3D
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Owsik, I., Kolarz, B.N. & Jezierska, J. Oxidation of 2,6-di-tert-butylphenols to Diphenoquinones Catalysed by Schiff Base-Cu(II) Systems Immobilized on Polymer Support. Catal Lett 107, 197–203 (2006). https://doi.org/10.1007/s10562-005-0006-6
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10562-005-0006-6