Abstract
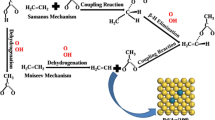
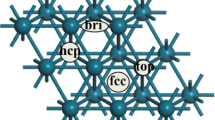
Adsorption and dehydrogenation of formic acid, hydrazine and isopropanol have been investigated using periodic density functional theory (DFT). All the intermediates and transition states have been optimized and the preferred reaction pathways have been found. The adsorption energies for the most stable mode of formic acid, hydrazine and isopropanol are 38.6 kJ/mol, 63.9 kJ/mol and 46.1 kJ/mol, respectively. The dehydrogenation mechanisms of formic acid, hydrazine and isopropanol on Pd(111) surface are proposed and calculated. According to the calculation results, dehydrogenation of formate is more favorable than those of other molecules/groups, and that can be an explanation for the high reactivity of formats in Pd catalyzed transfer hydrogenation.
Graphical Abstract










Similar content being viewed by others
References
Rajagopal S, Spatola AF (1995) J Org Chem 60:1347
Arcadi A, Gerichelli G, Chiarini M, Vico R, Zorzan D (2004) Eur J Org Chem 2004:3404
Cellier PP, Spindler JF, Taillefer M et al (2003) Tetrahedron Lett 44:7191
Ukisu Y (2008) Appl Catal A: Gen 349:229
Sydnes MO, Isobe M (2008) Tetrahedron Lett 49:1199
Wiener H, Blum J, Sasson Y (1991) J Org Chem 56:4481
Kopinke FD, Machenzie K, Koehler R, Georgi A (2004) Appl Catal A: Gen 271:119
Reddy PG, Baskaran S (2002) Tetrahedron Lett 43:1919
Tike MA, Mahajani VV (2006) Chem Eng J 123:31
German ED, Sheintuch M (2008) J Phys Chem C 112:14377
Srivastava GP, Weaire D (1987) Adv Phys 26:463
Marlo M, Milman V (2000) Phys Rev B 62:2899
Payne MC, Teter MP, Allan DC, Arias TA, Joannopoulos JD (1992) Rev Mod Phys 64:1045
Delley B (2000) J Chem Phys 113:7756
Gajdos M, Eichler A, Hafner J (2004) J Phys: Condens Matter 16:1141
Van Santen RA, Neurock M (1995) Catal Rev Sci Eng 37:557
Gil A, Clotet A, Ricart MJ, Kresse G, Garcia-Hernandez M, Roesch N, Sautet P (2003) Surf Sci 530:71
Perdew JP, Burke K, Ernzerhof M (1996) Phys Rev Lett 77:3865
Halgren TA, Lipscomb WN (1977) Chem Phys Lett 49:225
Vanderbilt D (1990) Phys Rev B 41:7892
Staykov A, Kamachi T, Ishihara T, Yoshizawa K (2008) J Phys Chem C 112:19501
Landolt-Boernstein (1976) Structure data of free polyatomic molecules, vol II/7., New SeriesSpringer, Berlin
Yue C, Lim KH (2009) Catal Lett 128:221
Tarmyshov KB, Mvller-Plathe F (2007) J Chem Phys 126:074702
Harcourt RD, Klapoetke TM, White PS (1998) Inorg Chim Acta 269:1
Feng G, Huo CF, Deng CM, Huang L, Li YW, Wang JG, Jiao HJ (2009) J Mol Catal A: Chem 304:58
Zheng T, Stacchiola D, Saldin DK, James J, Sholl DS, Tysoe WT (2005) Surf Sci 574:166
Rajagopal S, Spatola AF (1997) Appl Catal A: Gen 152:69
Cai S, Sohlberg K (2003) J Mol Catal A: Chem 193:157
Acknowledgments
We acknowledge the funding support by a grant from the National Natural Science Foundation of China (No. 20776127), the National Key Technology R&D Program (No. 2007BAI34B07).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhou, S., Qian, C. & Chen, X. Comparative Theoretical Study of Adsorption and Dehydrogenation of Formic Acid, Hydrazine and Isopropanol on Pd(111) Surface. Catal Lett 141, 726–734 (2011). https://doi.org/10.1007/s10562-011-0553-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-011-0553-y