Abstract
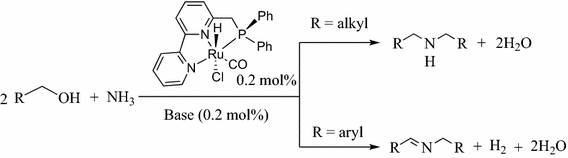
Efficient and selective direct synthesis of secondary amines from primary alcohols and ammonia with liberation of water has been achieved, with high turnover numbers and with no generation of waste. In case of benzylic alcohols, imines rather than amines are obtained. This atom economical, environmentally benign reaction is homogenously catalyzed by a well-defined bipyridine based Ru(II)-PNN pincer complex.
Graphical Abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Amines and their derivatives are a very important class of compounds in chemistry and biology. They are highly versatile building blocks for various organic synthetic targets and are used as dyes, color pigments, electrolytes, agricultural chemicals, herbicides, polymers, and functionalized materials [1]. In addition, they are essential pharmacophores in numerous biologically active compounds, and amine groups are present in vitamins, hormones, alkaloids, neurotransmitters, or natural toxics [2, 3]. Owing to their extensive applications, the development of versatile and efficient methods for the synthesis of secondary amines has attracted much interest in the area of catalysis. Because of the abundance and low price of ammonia, much attention has been focused on its use as a nitrogen source for organic synthesis [4, 5]. Recent reports on homogenous catalytic systems using ammonia as the substrate include palladium-catalyzed telomerization of butadiene and ammonia giving primary alkylamines [6], Pd-catalyzed allylic amination [7], copper- and palladium-catalyzed coupling reactions of ammonia with aryl halides to form arylamines [8–10], rhodium- and iridium-catalyzed reductive aminations of carbonyl compounds with ammonium formate and ammonia to afford primary alkylamines [11–13] and palladium-catalyzed arylation of ammonia to afford di- and triarylamines [10].
In the context of “green and sustainable chemistry” (GSC), the catalytic synthesis of amines from readily available alcohols using ammonia and generating no hazardous waste is of much current interest. In 2008, we reported the first direct homogenous catalytic selective amination of primary alcohols to primary amines using ammonia [14]. Later, the research groups of Vogt [15] and Beller [16] developed methods for the conversion of secondary alcohols to primary amines with ammonia. Catalytic methods for the synthesis of secondary amines starting from alcohols and amines were described by Williams [17, 18], Yamaguchi [19, 20], Beller [21, 22], Yus [23, 24] and Kempe [25, 26]. Multi alkylation of ammonia to get either secondary or tertiary amines was developed by Yamaguchi, Fujita and co-workers using an iridium complex as catalyst [27, 28]. Herein, we report the selective synthesis of secondary amines from alcohols and ammonia with liberation of water, with considerable turnover numbers (up to ~500) and with no generation of waste.
Our group has established several environmentally benign atom economical reactions, catalyzed by pincer complexes of ruthenium based on pyridine and acridine backbones, as outlined in recent reviews [29–33]. Of late we have developed the dearomatized bipyridine-based Ru(II)-pincer complex 5 (Fig. 1) and explored its catalytic activity towards the hydrogenation of amides [34], biomass-derived cyclic diesters [35], and urea derivatives [36] as well as organic carbonates, carbamates and formates [37]. The parent hydrido chloride pincer complex 2 catalyzes in the presence of catalytic base (in situ generation of the active dearomatized complex 5) several unique environmentally benign reactions such as conversion alcohols to carboxylic acid salts using water as the oxygen atom source [38], dehydrogenative cross-esterification reaction between primary and secondary alcohols [39], synthesis of pyrroles, pyridines and quinoline by dehydrogenative coupling of amino alcohols with secondary alcohols [40, 41] and catalytic coupling of nitriles with amines to selectively form imines under mild hydrogen pressure [42].
Recently we found that the pre-catalyst 3 analogue of 2, efficiently catalyzes in the presence of catalytic base the dehydrogenative coupling of primary alcohols with secondary amines to form tertiary amides [43], and the direct catalytic olefination of alcohols using sulfones, with liberation of H2 [44]. Herein we report the catalytic activity of the pre-catalyst 3 in bis-alkylation of ammonia with primary alcohols to get secondary amines, or imines (in the case of benzylic alcohols) selectively.
2 Results and Discussion
We have previously reported the preparation of the pincer complex 3, by reaction of 6-diphenylphosphinomethyl-2,2′-bipyridine [BPy(Ph)PNN] with [RuHCl(PPh3)3(CO)] in THF at 65 °C [43]. The structure of 3 was confirmed in the current study by a single-crystal X-ray diffraction study, using orange crystals grown from a solution of CH2Cl2:THF 4:1 (Fig. 2). Complex 3 exhibits a distorted octahedral geometry around the ruthenium center, with the CO ligand coordinated trans to the central nitrogen atom of the pincer system, and the hydride is located trans to the chloride.
X-ray structure of complex 3 (50 % probability level). Hydrogen atoms (except hydride) were omitted for clarity. Selected bond distances (Å): Ru1–N1 2.113(2), Ru1–N2 2.090(2), Ru1–P1 2.2403(8), Ru1–C24 1.839(4). Selected angles (deg): N2–Ru1–C24 175.39(12), N2–Ru1–H1 91.4(10), Cl1–Ru1–H1 175.1(10), N1–Ru1–P1 161.10(7)
Exploring the catalytic amination activity of complex 3, primary alcohols were reacted with ammonia in the presence of catalytic amounts of 3 (0.2 mol%) and KOtBu (0.2 mol%) in toluene (Scheme 1). Thus, heating a dry toluene solution of 1-butanol (10 mmol), complex 3 (0.2 mol%) and KOtBu (0.2 mol%) with NH3 (7 atm) at 135 °C resulted in 48 % conversion of 1-butanol to yield dibutylamine (34 % yield by GC), and 1-butylamine (14 %), after 18 h (Table 1, entry 1). When the reaction of 1-butanol was prolonged to 48 h the yield of dibutylamine increased to 88 % while 1-butylamine completely disappeared, and 7 % of butyl butyrate were also formed (Table 1, entry 2), indicating that complex 3 catalyzes the conversion of the initially formed primary amines to secondary amines under these conditions [14]. Exploring the scope of this reaction, various aliphatic primary alcohols were subjected to amination using 7 atm of NH3. Secondary amines were selectively formed, with no primary or tertiary amines being observed. Minor amounts of the corresponding esters were also formed in all cases, as a result of dehydrogenative coupling of alcohols (Table 1) [39, 45]. No amides were observed under these conditions, although complex 3 in the presence of catalytic base catalyzes the dehydrogenative coupling of primary alcohols with secondary amines to form tertiary amides [43]; however, this reaction requires efficient release of the generated H2, which is not possible in a reaction carried out in a sealed system under pressure. Reaction of 1-hexanol with ammonia yielded dihexylamine in 86 % yield together a small amount of hexyl hexanoate (3 % yield) and overall 90 % conversion of the alcohol after 48 h (Table 1, entry 3). The activated 2-methoxyethanol gave almost quantitative conversion to bis-2-methoxyethylamine in 85 % yield and 13 % yield of the corresponding ester, respectively (Table 1, entry 4). 3-methyl-1-butanol underwent amination and selectively yielded the corresponding secondary amine in moderate yield (79 %; Table 1, entry 7). Notably, reaction of benzylic alcohols resulted in formation of imines as the main products, with no secondary amine being formed. Thus, reaction of toluene solutions of benzyl alcohols (10 mmol) with ammonia (7 atm) and catalytic amounts of 3 and KOtBu (0.02 mmol) at 135 °C for 48 h, resulted in complete conversion, with selective formation of the corresponding imines and H2 (Table 1, entries 8–9). We have previously reported the dehydrogenative coupling of alcohols and amines (not ammonia) to form imines, catalyzed by a PNP-type Ru pincer complex [46].
A possible overall mechanistic scheme for the formation of secondary amines from primary alcohols and ammonia is as follows (Scheme 2): 1) formation of the primary amine via borrowing hydrogen strategy [29, 47]; an intermediate aldehyde formed by dehydrogenation of the alcohol reacts with ammonia to form an imine a (via water elimination from a hemiaminal intermediate), which undergoes hydrogenation. 2) a similar reaction of the primary amine, in competition with ammonia, with the intermediate aldehyde, to form an alkyl imine b which undergoes hydrogenation to form the secondary amine (Path A; in case of aliphatic systems). In the case of benzylic amines, the formed benzyl imine b does not undergo hydrogenation, perhaps because of its stabilization as a result of conjugation of the double bond with the arene moiety. This imine b can also be formed by addition of the primary amine to the initially formed imine a, followed by ammonia liberation (path B), although this is less likely under ammonia pressure.
3 Conclusions
In summary, we have developed a new atom-economical, environmentally benign catalytic system for the direct synthesis of secondary amines from alcohols and ammonia. The reaction is homogenously catalyzed by the bipyridine-based Ru(II)-PNN pincer complex 3 in the presence of an equimolar amount of base (relative to Ru). The reactions of ammonia with aliphatic alcohols gave secondary amines exclusively, while those of aromatic alcohols afforded imines selectively. Only 0.2 mol% catalyst is needed. The efficiency, selectivity, atom-economy and mild reaction conditions of this process make it attractive for the selective synthesis of secondary amines or imines from readily available, renewable alcohols.
4 Experimental
All experiments with metal complexes and phosphine ligands were carried out under N2 in a Vacuum Atmospheres glovebox equipped with a MO 40-2 inert gas purifier, or using standard Schlenk techniques. All non-deuterated solvents were refluxed over sodium/benzophenoneketyl and distilled under argon atmosphere. All solvents were degassed with argon and kept in the glove box over 4Å molecular sieves. CD2Cl2 was used as received. Most of the chemicals (alcohols) used in the catalytic reactions were purified according to standard procedures (vacuum distillation). GC analysis were carried out using a Carboxen 1000 column on a HP 690 series GC system or HP-5 cross linked 5 % phenylmethylsilicone column (30 m × 0.32 mm × 0.25 µm film thickness, FID) on a HP 6890 series GC system using m-xylene (1 mmol) as an internal standard.
4.1 Synthesis of RuHCl(CO)([BPy(Ph)PNN] (3)
The hydrido-chloride pincer complex 3 was prepared by a reaction of the ligand [BPy(Ph)PNN] with RuHCl(CO)(PPh3)3 according to a previously reported procedure from our group [43]. Crystals suitable for a single-crystal X-ray diffraction study were obtained from a mixture of CD2Cl2: THF (20 mg in 0.4: 0.1 mL) at −32 °C after several days (~10 days).
4.2 X-ray Crystal Structure Determination of 3
A crystal was mounted in a MiTeGen loop and flash frozen in a nitrogen stream at 120 K. Data were collected on a Bruker Apex-II CCD diffractometer generator equipped with a sealed tube with MoKα radiation (λ = 0.71073 Å) and a graphite monochromator. The structure was solved using direct methods with AUTOSTRUCTURE module based on F 2 [48, 49].
Chemical Formula: 2(C24H20ClN2OPRu).C4H8O, Orange, plate, 0.10 × 0.10 × 0.01 mm3, monoclinic, C2/c, a = 29.1301(11) Å, b = 12.5264(4) Å, c = 13.5058(5) Å, β = 108.826(3) deg., V = 4664.6(3) Å3, Z = 4, fw = 1111.92, D c = 1.583 Mg/m3, μ = 0.880 mm−1. Full matrix least-squares of refinement based on F 2 gave an agreement factor R = 0.0323 for data with I > 2σ(I) and R = 0.0599 for all data (4757 reflections) with a goodness-of-fit of 1.006. Idealized hydrogen atoms were placed and refined in the riding mode, with the exception of the hydride ligand H1-Ru, which was located in the difference map and refined independently.
4.3 General Procedure for the Catalytic Direct Amination of Alcohols to Amines
Complex 3 (0.02 mmol), KOtBu (0.02 mmol), an alcohol (10 mmol), and toluene (2 mL) were added to a 90 mL Fischer-Porter tube under an atmosphere of purified nitrogen in a Vacuum Atmospheres glovebox. The tube was taken out of the glovebox and was purged by three successive cycles of pressurization/venting with NH3 (20 psi), then pressurized with NH3 (7 atm). The solution was heated at 135 °C (bath temperature) with stirring for 48 h (Table 1). After cooling to room temperature, excess NH3 was vented off carefully and the products were analyzed by GC using m-xylene as an internal standard.
References
Lawrence SA (2005) Amines: synthesis, properties and applications. Cambridge University Press, Cambridge
Seewald N, Jakubke HD (2009) Peptides: chemistry and biology. Wiley, Weinheim, p 1
Hughes AB (ed.) (2009) Freeland in amino acids, peptides and proteins in organic chemistry. Vol. 1, Wiley, Weinheim, p 43
Roundhill DM (1992) Chem Rev 92:1
Willis MC (2007) Angew Chem Int Ed 46:3402
Prinz T, Driessen-Hölscher B (1999) Chem Eur J 5:2069
Nagano T, Kobayashi S (2009) J Am Chem Soc 131:4200
Lang F, Zewge D, Houpis IN, Volante RP (2001) Tetrahedron Lett 42:3251
Shen Q, Hartwig JF (2006) J Am Chem Soc 128:10028
Surry DS, Buchwald SL (2007) J. Am. Chem. Soc. 129:10354 and references cited therein
Kitamura M, Lee D, Hayashi S, Tanaka S, Yoshimura M (2002) J Org Chem 67:8685
Gross T, Seayad AM, Ahmad M, Beller M (2002) Org Lett 4:2055
Ogo S, Makihara N, Kaneko Y, Watanabe Y (2001) Organometallics 20:4903
Gunanathan C, Milstein D (2008) Angew Chem Int Ed 47:8661
Pingen D, Müller C, Vogt D (2010) Angew Chem Int Ed 49:8130
Imm S, Bähn S, Neubert L, Neumann H, Beller M (2010) Angew Chem Int Ed 49:8126
Watson AJA, Maxwell AC, Williams JMJ (2011) J Org Chem 76:2328
Saidi O, Blacker AJ, Farah MM, Marsden SP, Williams JMJ (2010) Chem Commun 46:1541
Kawahara R, Fujita K, Yamaguchi R (2011) Adv Synth Catal 353:1161
Yamaguchi R, Mingwen Z, Kawagoe S, Asai C, Fujita K (2009) Synthesis 1220
Bähn S, Imm S, Neubert L, Zhang M, Neumann H, Beller M (2011) ChemCatChem 3:1853
Hollmann D, Tillack A, Michalik D, Jackstell R, Beller M (2007) Chem Asian J 2:403
Martinez-Asencio A, Yus M, Ramon DJ (2011) Synthesis 2011:3730
Guillena G, Ramon DJ, Yus M (2010) Chem Rev 110:1611
Michlik S, Hille T, Kempe R (2012) Adv Synth Catal 354:847
Blank B, Michlik S, Kempe R (2009) Chem Eur J 15:3790
Yamaguchi R, Kawagoe S, Asai C, Fujita K (2008) Org Lett 10:181
Kawahara R, Fujita K, Yamaguchi R (2010) J Am Chem Soc 132:15108
Gunanathan C, Milstein D (2013) Science 341:249 and references therein
Gunanathan C, Milstein D (2011) Acc Chem Res 44:588
Milstein D (2010) Top Catal 53:915
Gunanathan C, Milstein D (2011) Top Organomet Chem 37:55
Balaraman E, Milstein D (2014) Top Organomet Chem 48:14
Balaraman E, Gnanaprakasam B, Shimon LJW, Milstein D (2010) J Am Chem Soc 132:16756
Balaraman E, Fogler E, Milstein D (2012) Chem Commun 48:1111
Balaraman E, Ben-David Y, Milstein D (2011) Angew Chem Int Ed 50:11702
Balaraman E, Gunanathan C, Zhang J, Shimon LJW, Milstein D (2011) Nature Chem 3:609
Balaraman E, Khaskin E, Leitus G, Milstein D (2013) Nature Chem 5:122
Srimani D, Balaraman E, Gnanaprakasam B, Ben-David Y, Milstein D (2012) Adv Synth Catal 354:2403
Srimani D, Ben-David Y, Milstein D (2013) Angew Chem Int Ed 52:4012
Srimani D, Ben-David Y, Milstein D (2013) Chem Commun 49:6632
Srimani D, Feller M, Ben-David Y, Milstein D (2012) Chem Commun 48:11853
Srimani D, Balaraman E, Hu P, Ben-David Y, Milstein D (2013) Adv Synth Catal 355:2525
Srimani D, Leitus G, Ben-David Y, Milstein D (2014) Angew Chem Int Ed 53:11092
Zhang J, Leitus G, Ben-David Y, Milstein D (2005) J Am Chem Soc 127:10840
Gnanaprakasam B, Zhang J, Milstein D (2010) Angew Chem Int Ed 49:1468
Hamid MHSA, Slatford PA, Williams JMJ (2007) Adv Synth Catal 349:1555
Sheldrick GM (1997) SHELXS-97, Program for Crystal Structure Solution. University of Göttingen, Göttingen
Sheldrick GM (1997) SHELXL-97, Program for Crystal Structure Refinement. University of Göttingen, Göttingen
Acknowledgments
This research was supported by the European Research Council under the FP7 framework (ERC No. 246837) and by the Israel Science Foundation. D. M. holds the Israel Matz Professorial Chair of Organic Chemistry. E. B thanks to the CSIR-NCL (Start-up Grant No. MLP028726).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Balaraman, E., Srimani, D., Diskin-Posner, Y. et al. Direct Synthesis of Secondary Amines From Alcohols and Ammonia Catalyzed by a Ruthenium Pincer Complex. Catal Lett 145, 139–144 (2015). https://doi.org/10.1007/s10562-014-1422-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-014-1422-2