Abstract
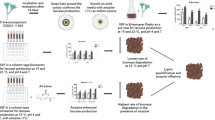
Laccase is a versatile enzyme that plays a major role in the remediation of various environmental pollutants. In this work, a thermo-tolerant halophilic Bacillus aquimaris AKRC02 was isolated from pulp and paper mill waste sludge for efficient laccase production. Various agro-industrial waste residues, including potato peel, banana peel, sawdust, pea peel, wheat bran, orange peel, and rice bran, were screened to produce laccase using a submerged fermentation process. Among these, rice bran supported the maximum laccase production (4.58 U/mL). The optimized environmental conditions (incubation time 120 h; 4.58 U/mL), 35 0C; 6.624 U/mL) and pH 7.0; 10.142 U/mL) and nutritional sources (glucose 1.0%; 14.164 U/mL and peptone 0.5%; 18.124 U/mL) significantly enhanced the laccase production. Purified laccase showed a specific activity and purification fold of 228.34 U/mg and 38.08, respectively. The purified enzyme showed a molecular weight of 65 kDa and high thermal stability at 45 0C for 8 h. In conclusion, the remarkable properties of the newly isolated bacterium may provide a significant opportunity for degrading environmental contaminants, making it an attractive biocatalyst for industrial applications.
Graphic Abstract









Similar content being viewed by others
Data Availability
Not applicable.
Abbreviations
- RSM :
-
Response surface methodology
- CCD :
-
Central composite design
- SmF :
-
Submerged fermentation
- 3 D :
-
Three dimensional
- SDS-PAGE :
-
Sodium dodecyl sulphate–polyacrylamide gel electrophoresis
- MSM :
-
Minimal salt media
- ARW :
-
Agro-residues wastes
- SEM :
-
Scanning electron microscope
- MBSS :
-
Mineral Basal Salt Solution
References
Kumar A, Chandra R (2020) Ligninolytic enzymes and its mechanisms for degradation of lignocellulosic waste in environment. Heliyon 6:e031702
Shraddha SR, Sehgal S, Kamthania M, Kumar A (2011) Laccase: microbial sources, production, purification, and potential biotechnological applications. Enzy Res Article ID. https://doi.org/10.4061/2011/217861
Mathur P, Sanyal D, Dey P (2021) Optimization of growth conditions for enhancing the production of microbial laccase and its application in treating antibiotic contamination in wastewater. 3 Biotech. https://doi.org/10.1007/s13205-020-02627-1
Chauhan PS, Goradia B, Saxena A (2017) Bacterial laccase: recent update on production and industrial applications. 3 Biotech. https://doi.org/10.1007/s13205-017-0955-7
Syahlan M, Syukri M, Rahman RA, Mohamad Z, Illias RMd, Mahmood NAN, Jaafar NR (2021) Optimization strategy for laccase immobilization on polyethylene terephthalate grafted with maleic anhydride electrospun nanofiber mat. Int J Biol Macromol 166:876–883
Walker TW, Kuch N, Meulen KAV, Clewett CFM, Huber GW, Fox BG, Dumesic JA (2020) Solid-state NMR studies of solvent-mediated, acid-catalyzed woody biomass pretreatment for enzymatic conversion of residual cellulose. ACS Sustainable Chem Eng 8(16):6551–6563
Barcelos Carolina A, Oka Asun M et al (2021) High-efficiency conversion of ionic liquid-pretreated woody biomass to ethanol at the pilot scale. ACS Sustainable Chem Eng 9(11):4042–4053
Aritomo Y, Naoki M, Masayuki S, Osamu S (2019) ACS Sustainable Chem Eng 7(12):10445–10451
Kuznetsov BA, Shumakovich GP, Koroleva OV, Yaropolov AI (2001) On applicability of laccase as label in the mediated and mediator lesse ectroimmuno assay: effect of distance on the direct electron transfer between laccase and electrode. Biosens Bioelectron 16:73–84
Muthukumar NP, Murugan S (2014) Production Purification and Application of Bacterial Laccase: A Review. Biotechnology (Faisalabad) 13(5):196–205. https://doi.org/10.3923/biotech.2014.196.205
Nyanhongo GS, Gomes J, Gubitz G, Zvauya J, Read S, Steiner W (2002) Production of laccase by a newly isolated strain of Trametes modest. Biores Technol 84:259–263
Singh AK, Kumar A, Chandra R 2020 Residual organic pollutants detected from pulp and paper industry wastewater and their toxicity on Triticum aestivum and Tubifex-tubifex worms. Materials Today: Proceedings. Doi: https://doi.org/10.1016/j.matpr.2020.10.862
Chauhan PS, Kumarasamy M, Sosnik A, Danino D (2019) Enhanced thermostability and anticancer efficacy in breast cancer cells, of laccase immobilized on Pluronic® stabilized nanoparticles. ACS Appl Mater Interfaces 11:39436–39448
Baral NR, Sundstrom ER, Das L, Gladden J et al (2019) Approaches for more efficient biological conversion of lignocellulosic feedstocks to biofuels and bioproducts. ACS Sustainable Chem Eng 7(10):9062–9079
Chauhan PS, Goradia B, Jha B (2018) Optimization and up-scaling of ionic liquid tolerant, thermo-alkali stable extracellular laccase from marine Staphylococcus arlettae S1–20 using tea waste. J Taiwan Inst Chem Eng 86:1–8
Singh B, Verma AK, Kumar P (2020) Catalytic thermolysis treatment of petroleum refinery wastewater collected from effluent treatment plant. Int J Chem Reactor Eng 18:5–6. https://doi.org/10.1515/ijcre-2019-0210
Ghosh P, Ghosh U (2017) Statistical optimization of laccase production by Aspergillus flavus PUF5 through submerged fermentation using agro-waste as cheap substrate. Acta Biologica Szegediensis 61:25–33
Parvez AM, Afzal MT, Hebb TGV, Schmid M 2020 Utilization of CO2 in thermochemical conversion of biomass for enhanced product properties: A review. J CO2 Util Doi: https://doi.org/10.1016/j.jcou.2020.101217
Cao L, Lin L, Sui H, Wang H, Zhang Z, Jiaoe N, Zhoug J (2021) Efficient extracellular laccase secretion via bio-designed secretory apparatuses to enhance bacterial utilization of recalcitrant lignin. Green Chem 23:2079–2094
Chauhan PS, Jha B (2018) Pilot scale production of extracellular thermo-alkali stable laccase from Pseudomonas sp. S2 using agro waste and its application in organophosphorus pesticides degradation. J Chem Technol Biotechnol 93(4):1022–1030
Hafid HS, Baharuddin AS, Mokhtar MN, Omar FN, Mohd Mohammed AP, Wakisaka M (2021) Enhanced laccase production for oil palm biomass delignification using biological pretreatment and its estimation at biorefinary scale. Biomass and Bioenergy 144:e105904
Abdelgalil SA, Soliman NA, Abo-Zaid GA et al (2021) Bioprocessing strategies for cost-effective large-scale production of bacterial laccase from Lysinibacillus macroides LSO using bio-waste. Int J Environ Sci Technol. https://doi.org/10.1007/s13762-021-03231-3
Wang F, Ling Xu, Zhao L, Ding Z, Ma H, Terry N (2019) Fungal laccase production from lignocellulosic agricultural wastes by solid-state fermentation: a Review. Microorganisms 7:665
Jovic J, Hao J, Kocic-Tanackov S et al (2020) Improvement of lignocellulosic biomass conversion by optimization of fungal ligninolytic enzyme activity and molasses stillage supplementation. Biomass Conv Bioref. https://doi.org/10.1007/s13399-020-00929-1
Coutinho de Lucas R, Brito de Oliveira T et al (2021) The profile secretion of Aspergillus clavatus: Different pre-treatments of sugarcane bagasse distinctly induces holocellulases for the lignocellulosic biomass conversion into sugar. Renewable Energy 165:748–757. https://doi.org/10.1016/j.renene.2020.11.072
Chen G, Dong J, Wan J et al (2021) Fiber characterization of old corrugated container bleached pulp with laccase and glycine pretreatment. Biomass Conv Bioref. https://doi.org/10.1007/s13399-020-01200-3
Cho SH, Jung S, Park YK, Tsang YF, Ryu C, Kwon EE 2020 Synergistic effects of CO2 on ex-situ catalytic pyrolysis of lignocellulosic biomass over a Ni/SiO2 catalyst. Journal of CO2 Utilization Doi: https://doi.org/10.1016/j.jcou.2020.101182
Kumar A, Chandra R (2021) Biodegradation and toxicity reduction of pulp paper mill wastewater by isolated laccase producing Bacillus cereus AKRC03. Cleaner Eng Technol 4:100193. https://doi.org/10.1016/j.clet.2021.100193
Chandra R, Singh R (2012) Decolourisation and detoxification of rayon grade pulp paper mill effluent by mixed bacterial culture isolated from pulp paper mill effluent polluted site. Biochem Eng J 61:49–58
Kumar A, Singh AK, Ahmad S, Chandra R (2020) Optimization of laccase production by Bacillus sp. strain AKRC01 in presence of agro-waste as effective substrate using response surface methodology. J Pure Appl Microbiol 14(1):351–362
Sneath PHA 1986 (editor) Bergey’s Manual of Systematic Bacteriology, Baltimore: Williams & Wilkins
Crowan and Steel's 1993 Manual for the Identification of Medical Bacteria. 3rd edn. Ed GI Barrow, RKA Feltham. (Pp 331; £40.) Cambridge University Press. ISBN 0–521–32611–7
Kapley A, Lampel K, Purohit HJ (2001) Rapid detection of Salmonella in water samples by multiplex PCR. Water Environ Res 73:461–465
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
O’Dwyer MH (1923) The hemicelluloses, III. The hemicellulose of American white oak. Biochemical Journal 17:501–509
Norman AG, Jenkins SH (1933) A new method for the determination of cellulose, based upon observations on the removal of lignin and other encrusting materials. Biochemical Journal 27:818–831
Arora DS, Chander M, Gill PK (2002) Involvement of lignin peroxidase, manganese peroxidase and laccase in degradation and selective ligninolysis of wheat straw. Int Biodeterior Biodegradation 50:115–120
Teigiserova DA, Bourgine J, Thomsen M (2021) Closing the loop of cereal waste and residues with sustainable technologies: an overview of enzyme production via fungal solid-state fermentation. Sustain Prod Consum 27:845–857
Muthukumarasamy NP, Jackson B, Raj AJ, Sevanan M (2015) Production of extracellular laccase from Bacillus subtilis MTCC 2414 using agro-residues as a potential substrate. Biochem Res Int Article ID 765190:9. https://doi.org/10.1155/2015/765190
Niladevi KN, Sukumaran RK, Prema P (2007) Utilization of rice straw for laccase production by Streptomyces psammoticus in solid-state fermentation. J Ind Microbiol Biotechnol 34(10):665–674
Singh AK, Kumar A, Chandra R (2020) Detection of refractory organic pollutants from pulp paper mill effluent and their toxicity on Triticum aestivum; Brassica campestris and Tubifex-tubifex. J Exp Biol Agric Sci 8(5):663–675
Antunes Barros AA, Cardoso APR, Rodrigues AC, Marinho da Silva CJS, Zille A (2019) Optimizing enzymatic dyeing of wool and leather. SN Applied Sciences 1:1232
Fatma G, Satinder KB, Tyagi RD, Rojan P, John M, Valero JR (2011) Parameter optimization for production of ligninolytic enzymes using agro-industrial wastes by response surface method. Biotechnol Bioprocess Eng 16:343–351
Kuddus M, Joseph B, Ramteke PW (2013) Production of laccase from newly isolated Pseudomonas putida and its application in bioremediation of synthetic dyes and industrial effluents. Biocatal Agric Biotechnol 2(4):333–338
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Sondhi S, Kaur R, Madan J (2021) Purification and characterization of a novel white highly thermo stable laccase from a novel Bacillus sp. MSK-01 having potential to be used as anticancer agent. Int J Biol Macromol 170:232–238
Xiao Y, Li J, Wu P, Ning N, Li J, Shen Y, Huang Q, Ni J (2021) An alkaline thermostable laccase from termite gut associated strain of Bacillus stratosphericus. Int J Biol Macromol 179:270–278
Kumar M, Kumar P, Das P, Solanki R, Kapur MK (2020) Potential applications of extracellular enzymes from Streptomyces spp. in various industries. Arch Microbiol 202:1597–1615
Senthivelan T, KanagarajRames J, Panda C, Narayani T (2019) Screening and production of a potential extracellular fungal laccase from Penicillium chrysogenum: Media optimization by response surface methodology (RSM) and central composite rotatable design (CCRD). Biotechnol Reports 23:e0034
Shrestha P, Joshi B, Joshi J, Malla R, Sreerama L 2016 Isolation and physicochemical characterization of laccase from Ganoderma lucidum-CDBT1 isolated from its native habitat in Nepal. BioMed research international article ID 3238909, 10 pages
Kiiskinen LL, Ratto M, Kruus K (2004) Screening for novel laccase-producing microbes. J Appl Microbiol 97:640–646
Tavakoli A, Hamzah A (2016) Characterization and evaluation of catechol oxygenases by twelve bacteria, isolated from oil contaminated soils in Malaysia. Biol J Microorg 5(20):71–80
Ornston LN, Stanier RY (1966) The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. J Biol Chem 241(16):3776–3786
Yoon J-H, Kim I-G, Kang KH, Tae-Kwang Oh, Park Y-H (2003) Bacillus marisflavi sp. nov. and Bacillus aquimaris sp. nov., isolated from sea water of a tidal flat of the Yellow Sea in Korea. Int J Syst Evol Microbiol 53:1297–1303
Shivanand P, Jayaraman G (2009) Production of extracellular protease from halotolerant bacterium, Bacillus aquimaris strain VITP4 isolated from Kumta coast. Process Biochem 44:1088–1094
Ngo HT, Nguyen TTN, Nguyen QM, Tran AV, Do HTV, Nguyen AH, Phan TN, Nguyen ATV (2016) Screening of pigmented Bacillus aquimaris SH6 from the intestinal tracts of shrimp to develop a novel feed supplement for shrimp. J Appl Microbiol 121:1357–1372
Kumar A, Singh AK, Chandra R (2020) Comparative analysis of residual organic pollutants from bleached and unbleached paper mill wastewater and their toxicity on Phaseolus aureus and Tubifex tubifex. Urban Water Journal 17(10):860–870
Khalid S, Irfan M, Shakir HA, Qazi JI (2017) Endoglucanase producing potential Bacillus species isolated from the gut of Lobeo rohita. J Mar Sci Technol 25(5):581–587
Agrawal K, Verma P (2020) Production optimization of yellow laccase from Strophari sp ITCC 8422 and enzyme-mediated depolymerization and hydrolysis of lignocellulosic biomass for biorefinery application. Biomass Conv Bioref. https://doi.org/10.1007/s13399-020-00869-w
Cheng T, Pandyaswargo AH, Onoda H (2020) Comparison of torrefaction and hydrothermal treatment as pretreatment technologies for rice husks. Energies 13(19):5158. https://doi.org/10.3390/en13195158
Niladevi KN, Prema P (2008) Immobilization of laccase from Streptomyces psammoticus and its application in phenol removal using packed bed reactor. World J Microbiol Biotechnol 24:1215–1222
Waghmare PR, Patil SM, Jadhav SL, Jeon BH, Govindwar SP (2018) Utilization of agricultural waste biomass by cellulolytic isolate Enterobacter sp. SUK-Bio Agric Natural Res 52(5):399–406
Liew CY, Husaini A, Hussain H, Muid S, Liew KC, Roslan HA (2011) Lignin biodegradation and ligninolytic enzyme studies during biopulping of Acacia mangium wood chips by tropical white-rot fungi. World J Microbiol Biotechnol 27(6):1457–1468
Huttermann A, Hamza AS, Chet I, Majcherczyk A, Fouad T, Badr A, Cohen R, Persky L, Hadar Y (2000) Recycling of agricultural wastes by white-rot fungi for the production of fodder for ruminants. Agro-Food Ind Hi-Tech 11:29–35
Carrillo-Nieves D, Saldarriaga-Hernandez S, Gutiérrez-Soto G et al (2020) Biotransformation of agro-industrial waste to produce lignocellulolytic enzymes and bioethanol with a zero waste. Biomass Conv Bioref. https://doi.org/10.1007/s13399-020-00738-6
Ahmad S, Pathak VV, Kothari R, Kumar A, Krishna SBN (2018) Optimization of nutrient stress using C. pyrenoidosa for lipid and biodiesel production in integration with remediation in dairy industry wastewater using response surface methodology. 3 Biotech 8(8):e326
Dalfard AB, Khajeh K, Soudi MR, Naderi-Manesh H, Ranjbar B, Sajedi RH (2006) Isolation and biochemical characterization of laccase and tyrosinase activities in a novel melanogenic soil bacterium. Enzyme Microb Technol 39:1409–1416
Mehandia S, Sharma SC, Arya SK (2020) Isolation and characterization of an alkali and thermostable laccase from a novel Alcaligenes faecalis and its application in decolorization of synthetic dyes. Biotechnol Reports 25:e00413. https://doi.org/10.1016/j.btre.2019.e00413
Sondhi S, Sharma P, George N et al (2015) An extracellular thermo-alkali-stable laccase from Bacillus tequilensis SN4, with a potential to biobleach softwood pulp. 3 Biotech 5:175–185
Navada KK, Kulal A (2020) Kinetic characterization of purified laccase from Trametes hirsuta: a study on laccase catalyzed biotransformation of 1,4-dioxane. Biotechnol Lett. https://doi.org/10.1007/s10529-020-03038-1
Campos PA, Levin LN, Wirth SA (2016) Heterologous production, characterization and dye decolorization ability of a novel thermostable laccase isoenzyme from Trametes trogii BAFC 463. Process Biochem 51:895–903
Acknowledgements
This work was supported by University Grant Commission, New Delhi, to Mr. Adarsh Kumar is also highly acknowledged, NFPwD (F No.01-01/2019-Sch.) fellowship to Mr. Ajay Kumar and the Department of Biotechnology New Delhi, Letter No. BT/PR18896/BCE/8/1372/2016 dated 28-03-2018 to Prof. Ram Chandra.
Author information
Authors and Affiliations
Contributions
Adarsh Kumar performed the experiments, drafting, editing the manuscript, Ajay Kumar Singh helped during the research work, and Muhammad Bilal editing and reviewing the manuscript. Ram Chandra supervised the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no any conflict of interest.
Ethics Approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumar, A., Singh, A.K., Bilal, M. et al. Sustainable Production of Thermostable Laccase from Agro-Residues Waste by Bacillus aquimaris AKRC02. Catal Lett 152, 1784–1800 (2022). https://doi.org/10.1007/s10562-021-03753-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-021-03753-y