Abstract
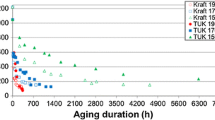
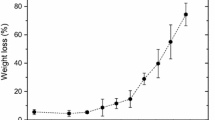
Recently, the existence of a relation between the rupture of 1,4-β-glycosidic bonds in the cellulose during thermal-ageing of paper/oil systems and the detection of methanol in the oil has been reported for the first time in this journal (Jalbert et al. 2007). The present study addresses the rate constants of the reaction for standard wood kraft papers, two immersed in inhibited naphthenic oil under air (paper/oil weight–volume ratio of 1:18) and one in non-inhibited paraffinic oil under nitrogen (paper/oil weight–volume ratio of 1:30). The isotherms in the range of 60–130 °C show that the initial rate of methanol production markedly increases with temperature and to a lesser extent with the moisture of the specimens (initially between 0.5 and 2.25% (w/w)), similarly to what is noted for the depolymerization through the Ekenstam’s pseudo-zero order model. The Arrhenius expression of the rate constants reveals linear relationships that confirm the dominance of a given mechanism in both cases. A very good agreement is also noted for the activation energy over the entirely paper/oil systems studied (106.9 ± 4.3 and 103.5 ± 3.7 kJ mol−1 for methanol and scissions, respectively). Furthermore, a comparison of the rate constants \( \left( {k_{{{\text{CH}}_{ 3} {\text{OH}}}} /k_{\text{scissions}} } \right) \) shows approximately constant values indicating an apparent yield for the methanol of about one-third molecule per every scission for the tests under air (0.27 ± 0.04 for Clupak HD75 and 0.37 ± 0.14 for Munksjö TH70) and even lower for the ones under N2 (0.12 ± 0.03 for Munksjö E.G.). As expected from a pseudo-zero order model, these values were shown to be consistent with a similar comparison of the amount of CH3OH and chain-end groups produced under specific time–temperature ageing conditions (168 h at 120 °C). Finally, an additional test carried out with unaged cellulose in contact with a fresh solution of methanol in oil (cellulose/oil weight–volume ratio of 1:18) shows that at equilibrium, over 58% of the species is lost from the solution due to penetration into the fibres. Such results reveal the importance of the species partitioning in establishing the true correspondence between the molecules of CH3OH produced and the scissions.







Similar content being viewed by others
References
Agarwal N, McKean WT, Gustafson RR (1991) Cellulose degradation kinetics in alkaline pulping. In: Proceedings of 6th international symposium on wood pulping chemistry, Melbourne, Australia, 213–220
Calvini P (2005) The influence of the leveling-off degree of polymerization on the kinetics of cellulose degradation. Cellulose 12:445–447. doi:10.1007/s10570-005-2206-z
Calvini P, Gorassini A (2006) On the rate of paper degradation: lessons from the past. Restaurator 27:275–290. doi:10.1515/REST.2006.275
Calvini P, Gorassini A, Merlani L (2008) On the kinetics of cellulose degradation: looking beyond the pseudo order rate equation. Cellulose 15:193–203. doi:10.1007/s10570-007-9162-8
Daruwalla EH, Narsian MG (1966) Detection and identification of acid-sensitive linkages in cellulose fibre substances. Tappi 49(3):106–111
Ekenstam A (1936) The behavior of cellulose in mineral acid solution: kinetic study of the decomposition of cellulose in acid solutions. Ber Deutschen Chem Gesellschaft 69:553
Emsley AM, Stevens GC (1994) Kinetics and mechanisms of the low-temperature degradation of cellulose. Cellulose 1:26–56. doi:10.1007/BF00818797
Emsley AM, Heywood RJ, Ali M, Eley CM (1997) On the kinetics of degradation of cellulose. Cellulose 4:1–5. doi:10.1023/A:1018408515574
Fabre J, Pichon A (1960) Deteriorating processes and products of paper in oil. Application to transformers, CIGRE report no. 137
Jalbert J, Gilbert R, Tétreault P, Morin B, Lessard-Déziel D (2007) Identification of a chemical indicator of the rupture of 1, 4-β-glycosidic bonds of cellulose in an oil-impregnated insulating paper system. Cellulose 14:295–309. doi:10.1007/s10570-007-9124-1
Karst D, Yang Y (2007) Effect of structure of large aromatic molecules grafted onto cellulose on hydrolysis of the glycosidic linkages. Macromol Chem Phys 208:784–791. doi:10.1002/macp.200600637
Knosp R, Fallah E (1961) Étude du papier imprégné d’huile. Bull LCIE 30:237L–244L
Kuhn W (1930) On the kinetics of reduction of high molecular weight chains. Ber Deutschen Chem Gesellschaft 63:1503
Sharples A (1971) Acid hydrolysis and alcoholysis. In: Bikales NM, Segal I (eds) High polymer, degradation of cellulose and its derivatives, part V, 2nd edn. Wiley-Interscience, New York
Whitmore PM, Bogaard J (1994) Determination of the cellulose scission route in the hydrolytic and oxidative degradation of paper. Restaurator 15:26–45
Zou X, Uesaka T, Gurnagul N (1996) Prediction of paper permanence by accelerated aging I. Kinetic of the aging process. Cellulose 3:243–267. doi:10.1007/BF02228805
Acknowledgments
The authors sincerely acknowledge P. Gervais, DESTT TransÉnergie, Hydro-Québec, for his invaluable support during the project. Special mention is made of the financial support of Électricité de France, without whom the study of the system under nitrogen could not have been completed. The authors would also like to acknowledge M. Dahlund, from ABB Transformers, who kindly provided some sheets of Munksjö Thermo 70 insulating paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gilbert, R., Jalbert, J., Tétreault, P. et al. Kinetics of the production of chain-end groups and methanol from the depolymerization of cellulose during the ageing of paper/oil systems. Part 1: Standard wood kraft insulation. Cellulose 16, 327–338 (2009). https://doi.org/10.1007/s10570-008-9261-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-008-9261-1