Abstract
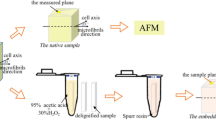
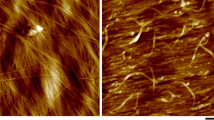
For more than 10 years epidermal cell layers from onion scales have been used as a model system to study the relationship between cellulose orientation, cell growth and tissue mechanics. To bring such analyses to the nanoscale, we have developed a procedure for preparing epidermal peels of onion scales for atomic force microscopy to visualize the inner surface (closest to the plasma membrane) of the outer epidermal wall, with minimal disturbance and under conditions very close to the native state of the cell wall. The oriented, multilayer distribution of cellulose microfibrils, approximately ~3 nm wide, is readily observed over extended lengths, along with other features such as the distribution of matrix substances between and on top of microfibrils. The microfibril orientation and alignment appear more dispersed in younger scales compared with older scales, consistent with reported values for mechanical and growth anisotropy of whole epidermal sheets. These results open the door to future work to relate cell wall structure at the nm scale with larger-scale tissue properties such as growth and mechanical behaviors and the action of cell wall loosening agents to induce creep of primary cell walls.





Similar content being viewed by others
References
Albersheim P, Darvill A, Roberts K, Sederoff R, Staehelin A (2011) Plant cell walls. Garland, NY, pp 1–430
Baskin T (2005) Anisotropic expansion of the plant cell wall. Annu Rev Cell Dev Biol 21:203–222
Baskin T, Gu Y (2012) Making parallel lines meet: transferring information from microtubules to extracellular matrix. Cell Adh Migr 6:1–5
Bootten TJ, Harris PJ, Melton LD, Newman RH (2004) Solid-state 13C-NMR spectroscopy shows that the xyloglucans in the primary cell walls of mung bean (Vigna radiata L.) occur in different domains: a new model for xyloglucan-cellulose interactions in the cell wall. J Exp Bot 55:571–583
Carpita NC, Gibeaut DM (1993) Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J 3:1–30
Cavalier DM, Lerouxel O, Neumetzler L, Yamauchi K, Reinecke A, Freshour G, Zabotina OA, Hahn MG, Burgert I, Pauly M, Raikhel NV, Keegstra K (2008) Disrupting two Arabidopsis thaliana xylosyltransferase genes results in plants deficient in xyloglucan, a major primary cell wall component. Plant Cell 20:1519–1537
Chafe SC, Wardrop AB (1972) Fine structural observations on the epidermis. I. The epidermal cell wall. Planta 278:269–278
Chen L, Wilson RH, McCann MC (1997) Investigation of macromolecule orientation in dry and hydrated walls of single onion epidermal cells by FTIR microspectroscopy. J Mol Struct 409:257–260
Cosgrove DJ (1993) Wall extensibility: its nature, measurement, and relationship to plant cell growth. New Phytol 124:1–23
Cosgrove DJ (2001) Wall structure and wall loosening. A look backwards and forwards. Plant Physiol 125:131–134
Cosgrove DJ (2005) Growth of the plant cell wall. Nat Rev Mol Cell Biol 6:850–861
Cosgrove DJ (2011) Measuring in vitro extensibility of growing plant cell walls. Methods Mol Biol 715:291–303
Crowell EF, Timpano H, Desprez T, Franssen-Verheijen T, Emons A-M, Höfte H, Vernhettes S (2011) Differential regulation of cellulose orientation at the inner and outer face of epidermal cells in the Arabidopsis hypocotyl. Plant Cell 23:2592–2605
Davies LM, Harris PJ (2003) Atomic force microscopy of microfibrils in primary cell walls. Planta 217:283–289
Dick-Perez M, Zhang Y, Hayes J, Salazar A, Zabotina OA, Hong M (2011) Structure and interactions of plant cell-wall polysaccharides by two- and three-dimensional magic-angle-spinning solid-state NMR. Biochemistry 50:989–1000
Ding SY, Himmel ME (2006) The maize primary cell wall microfibril: a new model derived from direct visualization. J Agric Food Chem 54:597–606
Ding SY, Liu YS, Zeng Y, Himmel ME, Baker JO, Bayer EA (2012) How does plant cell wall nanoscale architecture correlate with enzymatic digestibility? Science 338:1055–1060
Eisinger W, Croner L, Taiz L (1983) Ethylene-induced lateral expansion in etiolated pea stems. Kinetics, cell wall synthesis, and osmotic potential. Plant Physiol 73:407–412
Elazzouzi-Hafraoui S, Nishiyama Y, Putaux JL, Heux L, Dubreuil F, Rochas C (2008) The shape and size distribution of crystalline nanoparticles prepared by acid hydrolysis of native cellulose. Biomacromolecules 9:57–65
Fernandes AN, Thomas LH, Altaner CM, Callow P, Forsyth VT, Apperley DC, Kennedy CJ, Jarvis MC (2011) Nanostructure of cellulose microfibrils in spruce wood. Proc Natl Acad Sci USA 108:E1195–E1203
Fujino T, Sone Y, Mitsuishi Y, Itoh T (2000) Characterization of cross-links between cellulose microfibrils, and their occurrence during elongation growth in pea epicotyl. Plant Cell Physiol 41:486–494
Ha MA, Apperley DC, Jarvis MC (1997) Molecular rigidity in dry and hydrated onion cell walls. Plant Physiol 115:593–598
Hayashi T (1989) Xyloglucans in the primary cell wall. Annu Rev Plant Physiol Plant Mol Biol 40:139–168
Hepworth DG, Bruce DM (2004) Relationships between primary plant cell wall architecture and mechanical properties for onion bulb scale epidermal cells. J Texture Stud 35:586–602
Kennedy CJ, Cameron GJ, Sturcova A, Apperley DC, Altaner C, Wess TJ, Jarvis MC (2007) Microfibril diameter in celery collenchyma cellulose: X-ray scattering and NMR evidence. Cellulose 14:235–246
Kerstens S, Verbelen J (2003) Cellulose orientation at the surface of the Arabidopsis seedling. Implications for the biomechanics in plant development. J Struct Biol 144:262–270
Kirby AR, Gunning AP, Waldron KW, Morris VJ, Ng A (1996) Visualization of plant cell walls by atomic force microscopy. Biophys J 70:1138–1143
Kutschera U (2008) The growing outer epidermal wall: design and physiological role of a composite structure. Ann Bot 101:615–621
Liu YS, Baker JO, Zeng Y, Himmel ME, Haas T, Ding SY (2011) Cellobiohydrolase hydrolyzes crystalline cellulose on hydrophobic faces. J Biol Chem 286:11195–11201
Loodts J, Tijskens E, Wei CF, Vanstreels E, Nicolai B, Ramon H (2006) Micromechanics: simulating the elastic behavior of onion epidermis tissue. J Texture Stud 37:16–34
Marga F, Grandbois M, Cosgrove DJ, Baskin TI (2005) Cell wall extension results in the coordinate separation of parallel microfibrils: evidence from scanning electron microscopy and atomic force microscopy. Plant J 43:181–190
McCann MC, Wells B, Roberts K (1990) Direct visualization of cross-links in the primary plant cell wall. J Cell Sci 96:323–334
Ng A, Parker ML, Parr AJ, Saunders PK, Smith AC, Waldron KW (2000) Physicochemical characteristics of onion (Allium cepa L.) tissues. J Agric Food Chem 48:5612–5617
Nishiyama Y (2009) Structure and properties of the cellulose microfibril. J Wood Sci 55:241–249
Paredez AR, Somerville CR, Ehrhardt DW (2006) Visualization of cellulose synthase demonstrates functional association with microtubules. Science 312:1491–1495
Park YB, Cosgrove DJ (2012a) A revised architecture of primary cell walls based on biomechanical changes induced by substrate-specific endoglucanases. Plant Physiol 158:1933–1943
Park YB, Cosgrove DJ (2012b) Changes in cell wall biomechanical properties in the xyloglucan-deficient xxt1/xxt2 mutant of Arabidopsis. Plant Physiol 158:465–475
Qian M, Wells DM, Jones A, Becker A (2010) Finite element modelling of cell wall properties for onion epidermis using a fibre-reinforced hyperelastic model. J Struct Biol 172:300–304
Savaldi-Goldstein S, Peto C, Chory J (2007) The epidermis both drives and restricts plant shoot growth. Nature 446:199–202
Scheller HV, Ulvskov P (2010) Hemicelluloses. Annu Rev Plant Biol 61:263–289
Scott A, Wyatt S, Tsou P-L, Robertson D, Allen N (1999) Model system for plant cell biology: GFP imaging in living onion epidermal cells. Biotechniques 1132:1125–1132
Su C, Hu Y, Erina N, Slade A (2010) Quantitative mechanical mapping of biomolecules and cells in fluid. MRS Proc 1261:1261-U01-05
Suslov D, Verbelen JP (2006) Cellulose orientation determines mechanical anisotropy in onion epidermis cell walls. J Exp Bot 57:2183–2192
Suslov D, Verbelen JP, Vissenberg K (2009) Onion epidermis as a new model to study the control of growth anisotropy in higher plants. J Exp Bot 60:4175–4187
Szymanski DB, Cosgrove DJ (2009) Dynamic coordination of cytoskeletal and cell wall systems during plant cell morphogenesis. Curr Biol 19:R800–R811
Taiz L (1984) Plant cell expansion: regulation of cell wall mechanical properties. Annu Rev Plant Physiol 35:585–657
Terashima N, Kitano K, Kojima M, Yoshida M, Yamamoto H, Westermark U (2009) Nanostructural assembly of cellulose, hemicellulose, and lignin in the middle layer of secondary wall of ginkgo tracheid. J Wood Sci 55:409–416
Thimm JC, Burritt DJ, Ducker WA, Melton LD (2000) Celery (Apium graveolens L.) parenchyma cell walls examined by atomic force microscopy: effect of dehydration on cellulose microfibrils. Planta 212:25–32
Vanstreels E, Alamar AC, Verlinden BE, Enninghorst A, Loodts JKA, Tijskens E, Ramon H, Nicolai BM (2005) Micromechanical behaviour of onion epidermal tissue. Postharvest Biol Technol 37:163–173
Wilson RH, Smith AC, Kacurakova M, Saunders PK, Wellner N, Waldron KW (2000) The mechanical properties and molecular dynamics of plant cell wall polysaccharides studied by Fourier-transform infrared spectroscopy. Plant Physiol 124:397–405
Acknowledgments
This work was supported as part of The Center for LignoCellulose Structure and Formation, an Energy Frontier Research Center funded by the US Department of Energy, Office of Science, Basic Energy Sciences under Award # DE-SC0001090. We thank Mr. Ed Wagner for technical help as well as Dr. Seong H. Kim and Dr. Yong Bum Park for discussion and advice.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, T., Mahgsoudy-Louyeh, S., Tittmann, B. et al. Visualization of the nanoscale pattern of recently-deposited cellulose microfibrils and matrix materials in never-dried primary walls of the onion epidermis. Cellulose 21, 853–862 (2014). https://doi.org/10.1007/s10570-013-9996-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-013-9996-1