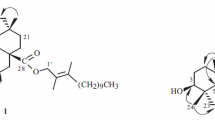
One new sterol, (3β,21α,22a)-stigmast-5-ene-3,21,22-triol (1), together with 36 compounds (2–37), were isolated from the stems of Aphanamixis grandifolia. The structure of 1 was elucidated by physical, chemical, and spectroscopic methods, including 1D and 2D NMR and HR-ESI-MS. Besides, 33 compounds (2, 4, 5, 7–10, 12–30, and 32–37) were isolated from the Aphanamixis genus for the first time, and 15 was isolated from the plant for the first time.

Similar content being viewed by others
References
S. K. Chen, Flora of China, Science, Beijing, 43, 1997, 77 pp.
J. Polonsky, Z. Varon, B. Arnoux, C. Pascard, G. R. Pettit, and J. H. Schmidt, J. Am. Chem. Soc., 100, 7731 (1978).
M. Nishizawa, A. Inoue, Y. Hayashi, S. Sastrapradja, S. Kosela, and T. Iwashita, J. Org. Chem., 49, 3660 (1984).
Q. Liu, C. J. Chen, X. Shi, L. Zhang, H. J. Chen, and K. Gao, Chem. Pharm. Bull., 58, 1431 (2010).
Y. Zhang, J. S. Wang, J. Luo, and L. Y. Kong, Chem. Pharm. Bull., 59, 282 (2011).
A. Astulla, Y. Hirasawa, A. Rahman, I. Kusumawati, W. Ekasari, A. Widyawaruyanti, N. C. Zaini, and H. Morita, Nat. Prod. Commun., 6, 323 (2011).
J. S. Wang, Y. Zhang, X. B. Wang, D. D. Wei, J. Luo, J. G. Luo, M. H. Yang, H. Q. Yao, H. B. Sun, and L. Y. Kong, Tetrahedron Lett., 53, 1705 (2012).
V. F. Rodrigues, H. M. Carmo, R. Braz Filho, L. Mathias, and I. J. C. Vieira, Nat. Prod. Commun., 5, 179 (2010).
H. Kawachi, R. Tanaka, M. Hirano, K. Igarashi, and H. Ooshima, J. Chem. Eng. Jpn., 39, 869 (2006).
M. Dell Gareca, P. Monaco, and L. Revitera, J. Nat. Prod., 53, 1430 (1990).
D. A. Mulholland, K. McFarland, and M. Randrianarivelojosia, Biochem. Syst. Ecol., 34, 365 (2006).
J. R. Li, X. F. Gao, T. M. Ai, and Y. Y. Zhao, China J. Chin. Mater. Med., 27, 40 (2002).
T. Iijima, Y. Yaoita, and M. Kikuchi, Chem. Pharm. Bull., 51, 545 (2003).
X. M. Tian, S. Z. Chen, P. F. Tu, and L. D. Lei, China J. Chin. Mater. Med., 33, 2204 (2008).
M. Dong and T. M. Guang, Nat. Prod. Res. Dev., 14, 34 (2002).
J. Q. Huang, J. Z. Yang, Q. C. Xue, L. Yu, and D. M. Zhang, China J. Chin. Mater. Med., 34, 1114 (2009).
M. S. Ali, M. K. Pervez, M. Saleem, and F. Ahmed, Nat. Prod. Res., 17, 301 (2003).
Z. Zhang, W. Zhang, Y. P. Ji, Y. Zhao, C. G. Wang, and J. F. Hua, Phytochemistry, 71, 693 (2010).
K. N. Asha, R. Chowdhury, C. M. Hasan, and M. A. Rashid, Acta Pharm., 54, 57 (2004).
M. C. Wu, C. F. Peng, I. S. Chen, and I. L. Tsai, J. Nat. Prod., 74, 976 (2011).
P. Tane, J. F. Ayafor, B. L. Sondengam, and J. D. Connolly, Phytochemistry, 29, 1004 (1990).
N. Ullah, S. Ahmad, E. Anis, P. Mohammad, H. Rabnawaz, and A. Malik, Phytochemistry, 50, 147 (1999).
S. F. Fonseca, L. T. Nielsen, and E. A. Rtiveda, Phytochemistry, 18, 1703 (1979).
S. S. Moon, A. A. Rahman, J. Y. Kim, and S. H. Kee, Bioor. Med. Chem., 16, 7264 (2008).
L. Li and N. P. Seeram, J. Agric. Food Chem., 59, 7708 (2011).
T. S. Wu, J. H. Yeh, and P. L. Wu, Phytochemistry, 40, 121 (1995).
S. G. Guan, W. B. Yu, and S. H. Guan, Lishizhen Med. Mater. Med. Res., 21, 905 (2010).
M. A. Ouyang, P. Q. Chen, and S. B. Wang, Nat. Prod. Res., 21, 769 (2007).
Y. M. Liu, G. Y. Liang, and B. X. Xu, Nat. Prod. Res. Dev., 15, 15 (2003).
H. G. Xie, H. W. Zhang, J. Zhang, L. Z. Xu, and Z. M. Zou, Chin. J. Nat. Med., 5, 193 (2007).
M. Kikuchi, Y. Yaoita, and M. Kikuchi, Helv. Chim. Acta, 91, 1857 (2008).
L. Chen, S. Izumi, D. I. Ito, T. Iwaeda, R. Utsumi, and T. Hirata, Chem. Lett., 3, 205 (1996).
A. Arfaoui, T. Ben Ayed, and H. Amri, J. Soc. Chim. Tunisie, 10, 1 (2008).
J. C. Bertrand and J. George, Magn. Reson. Chem., 30, 571 (1992).
G. F. Bannikov, G. A. Nikiforov, and V. V. Ershov, Izv. Akad. Nauk SSSR, Ser. Khim., 1408 (1985).
S. W. Yang and G. A. Cordell, J. Nat. Prod., 60, 44 (1997).
J. McNulty, J. J. Nair, S. Cheekoori, V. Larichev, A. Capretta, and A. J. Robertson, Chem.-Eur. J., 12, 9314 (2006).
B. S. Moore, K. Poralla, and H. G. Floss, J. Am. Chem. Soc., 115, 5267 (1993).
C. J. O’Connor, D. J. McLennan, D. J. Calvert, and A. S. H. Mitha, Aust. J. Chem., 40, 677 (1987).
A. C. Gonzalez-Baro, B. S. Parajon-Costa, C. A. Franca, and R. Pis-Diez, J. Mol. Struct., 889, 204 (2008).
Acknowledgment
The work was supported by the NCET Foundation program, NSFC (30725045 and 81102778) and partially supported by the Global Research Network for Medicinal Plants (GRNMP) and King Saud University, the Shanghai Leading Academic Discipline Project (B906), FP7-PEOPLE-IRSES-2008 (TCMCANCER Project 230232), the Key Laboratory of Drug Research for Special Environments, PLA, the Shanghai Engineering Research Center for the Preparation of Bioactive Natural Products (10DZ2251300), and the Scientific Foundation of Shanghai China (09DZ1972200, 09DZ1975700, 09DZ1971500, 10DZ1971700).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 3, May–June, 2013, pp. 415–419.
Rights and permissions
About this article
Cite this article
Zeng, Q., Ye, J., Ren, J. et al. Chemical Constituents from Aphanamixis grandifolia. Chem Nat Compd 49, 486–492 (2013). https://doi.org/10.1007/s10600-013-0644-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-013-0644-7