Summary
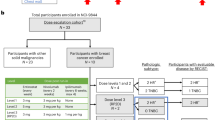
Twelve patients with metastatic breast cancer previously exposed to taxanes were treated on a Phase II trial with ixabepilone. Eligible patients had histologically confirmed metastatic breast cancer with measurable disease by Response Evaluation Criteria in Solid Tumors (RECIST), and adequate hematopoietic, renal, and hepatic function. Ixabepilone 8 mg/m2/day was given intravenously daily for 3 days for the first 3-week cycle and increased to 10 mg/m2/day for subsequent cycles if patients did not have hematologic or other toxicity after the first cycle. Patients continued treatment until progressive disease or unacceptable toxicity. Three, 29, and 33 of 65 cycles administered were at the 7 mg/m2, 8 mg/m2 and 10 mg/m2 dose levels respectively. Grade 4 leukopenia (n=1), grade 3 neutropenia (n=2), grade 2 neuropathy (n=3), and grade 2 transaminase elevation (n=2) were the most notable toxicities. Ten patients had stable disease for at least 6 weeks. No complete or partial responses were observed in 12 evaluable patients treated with ixabepilone daily for 3 days. Although ixabepilone was well-tolerated, the dose of 8–10 mg/m2 daily for 3 days is not an effective therapy in metastatic breast cancer previously exposed to taxanes.
Similar content being viewed by others
References
Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ (2006) Cancer statistics. CA Cancer J Clin 56:106–130
Chan S, Friedrichs K, Noel D, Pinter T, Van Belle S, Vorobiof D, Duarte R, Gil GM, Bodrogi I, Murray E, Yelle L, von Minckwitz G, Korec S, Simmonds P, Buzzi F, Gonzalez Mancha R, Richardson G, Walpole E, Ronzoni M, Murawsky M, Alakl M, Riva A, Crown J (1999) Prospective randomized trial of docetaxel versus doxorubicin in patients with metastatic breast cancer. J Clin Oncol 17:2341–2354
Gianni L, Munzone E, Capri G, Villani F, Spreafico C, Tarenzi E, Fulfaro F, Caraceni A, Martini C, Laffranchi A et al (1995) Paclitaxel in metastatic breast cancer: a trial of two doses by a 3-hour infusion in patients with disease recurrence after prior therapy with anthracyclines. J Natl Cancer Inst 87:1169–1175
Jones SE, Erban J, Overmoyer B, Budd GT, Hutchins L, Lower E, Laufman L, Sundaram S, Urba WJ, Pritchard KI, Mennel R, Richards D, Olsen S, Meyers ML, Ravdin PM (2005) Randomized phase III study of docetaxel compared with paclitaxel in metastatic breast cancer. J Clin Oncol 23:5542–5551
Nabholtz JM, Senn HJ, Bezwoda WR, Melnychuk D, Deschenes L, Douma J, Vandenberg TA, Rapoport B, Rosso R, Trillet-Lenoir V, Drbal J, Molino A, Nortier JW, Richel DJ, Nagykalnai T, Siedlecki P, Wilking N, Genot JY, Hupperets PS, Pannuti F, Skarlos D, Tomiak EM, Murawsky M, Alakl M, Aapro M (1999) Prospective randomized trial of docetaxel versus mitomycin plus vinblastine in patients with metastatic breast cancer progressing despite previous anthracycline-containing chemotherapy. 304 Study Group. J Clin Oncol 17:1413–1424
Kowalski RJ, Giannakakou P, Hamel E (1997) Activities of the microtubule-stabilizing agents epothilones A and B with purified tubulin and in cells resistant to paclitaxel (Taxol(R). J Biol Chem 272:2534–2541
Lee FY, Borzilleri R, Fairchild CR, Kim SH, Long BH, Reventos-Suarez C, Vite GD, Rose WC, Kramer RA (2001) BMS-247550: a novel epothilone analog with a mode of action similar to paclitaxel but possessing superior antitumor efficacy. Clin Cancer Res 7:1429–1437
Goodin S, Kane MP, Rubin EH (2004) Epothilones: mechanism of action and biologic activity. J Clin Oncol 22:2015–2025
Piccart MJ, Klijn J, Paridaens R, Nooij M, Mauriac L, Coleman R, Bontenbal M, Awada A, Selleslags J, Van Vreckem A, Van Glabbeke M (1997) Corticosteroids significantly delay the onset of docetaxel-induced fluid retention: final results of a randomized study of the European Organization for Research and Treatment of Cancer Investigational Drug Branch for Breast Cancer. J Clin Oncol 15:3149–3155
Mani S, McDaid H, Hamilton A, Hochster H, Cohen MB, Khabelle D, Griffin T, Lebwohl DE, Liebes L, Muggia F, Horwitz SB (2004) Phase I clinical and pharmacokinetic study of BMS-247550, a novel derivative of epothilone B, in solid tumors. Clin Cancer Res 10:1289–1298
Abraham J, Agrawal M, Bakke S, Rutt A, Edgerly M, Balis FM, Widemann B, Davis L, Damle B, Sonnichsen D, Lebwohl D, Bates S, Kotz H, Fojo T (2003) Phase I trial and pharmacokinetic study of BMS-247550, an epothilone B analog, administered intravenously on a daily schedule for five days. J Clin Oncol 21:1866–1873
Wiernik PH, Schwartz EL, Strauman JJ, Dutcher JP, Lipton RB, Paietta E (1987) Phase I clinical and pharmacokinetic study of taxol. Cancer Res 47:2486–2493
Zhuang SH, Menefee M, Kotz H, Agrawal M, Poruchynsky M, Hung E, Zhan Z, Linehan WM, Bates SE, Fojo T (2004) A phase II clinical trial of BMS-247550 (ixabepilone), a microtubule-stabilizing agent in renal cell cancer. J Clin Oncol, 22:4550 (Abstracts 4550)
Thomas E, Tabernero J, Fornier M, Fumoleau P, Lluch A, Viens P, Vahdat L, Conte P, Peck R, Martin M (2003) A phase II study of the epothilone B analog BMS-247550 in patients (pts) with taxane-resistant metastatic breast cancer (MBC). J Clin Oncol, 22:8 (Abstracts 30)
Smaletz O, Galsky M, Scher HI, DeLaCruz A, Slovin SF, Morris MJ, Solit DB, Davar U, Schwartz L, Kelly WK (2003) Pilot study of epothilone B analog (BMS-247550) and estramustine phosphate in patients with progressive metastatic prostate cancer following castration. Ann Oncol 14:1518–1524
Roche H, Cure H, Bunnell C, Cognetti F, Mauriac E, Perez E, Miller K, Sparano R, Peck R, Yelle L (2003) A phase II study of epothilone analog BMS-247550 in patients (pts) with metastatic breast cancer (MBC) previously treated with an anthracycline. J Clin Oncol, 22:69 (Abstracts 69)
Pavlick A, Millward M, Farrell K, Hamilton A, Broseus A, Haas N, Shore T, Jacquotte A, Colevas D, Muggia F (2004) A phase II study of epothilone B analog (EpoB)-BMS 247550 (NSC#710428) in stage IV malignant melanoma (MM). J Clin Oncol, 22:7542 (Abstracts 7542)
Okuno S, Maples WJ, Mahoney MR, Fitch T, Stewart J, Fracasso PM, Kraut M, Ettinger DS, Dawkins F, Erlichman C (2005) Evaluation of epothilone B analog in advanced soft tissue sarcoma: a phase II study of the phase II consortium. J Clin Oncol 23:3069–3073
Low JA, Wedam SB, Lee JJ, Berman AW, Brufsky A, Yang SX, Poruchynsky MS, Steinberg SM, Mannan N, Fojo T, Swain SM (2005) Phase II clinical trial of ixabepilone (BMS-247550), an epothilone B analog, in metastatic and locally advanced breast cancer. J Clin Oncol 23:2726–2734
Llombart Cussac A, Baselga J, Manikhas G, Kubista E, Steger G, Galbraith SM, Sullivan MA, Zerba K, Lee H, Gianni L (2005) Phase II genomics study in patients receiving ixabepilone as neoadjuvant treatment for breast cancer (BC): Preliminary efficacy and safety data. J Clin Oncol, 23:586 (Abstracts 586)
Hussain M, Faulkner J, Vaishampayan U, Lara P, Petrylak D, Colevas D, Sakr W, Crawford ED (2004) Epothilone B (Epo-B) analogue BMS-247550 (NSC #710428) administered every 21 days in patients (pts) with hormone refractory prostate cancer (HRPC). A Southwest Oncology Group Study (S0111). J Clin Oncol, 22:4510 (Abstracts 4510)
Eng C, Kindler HL, Nattam S, Ansari RH, Kasza K, Wade-Oliver K, Vokes EE (2004) A phase II trial of the epothilone B analog, BMS-247550, in patients with previously treated advanced colorectal cancer. Ann Oncol 15:928–932
Chen T, Molina A, Moore S, Goel S, Desai K, Hamilton A, Griffin T, Colevas AD, Mani S, Muggia F (2004) Epothilone B analog (BMS-247550) at the recommended phase II dose (RPTD) in patients (pts) with gynecologic (gyn) and breast cancers. J Clin Oncol, 22:2115 (Abstracts 2115)
Zhuang SH, Agrawal M, Edgerly M, Bakke S, Kotz H, Thambi P, Rutt A, Balis FM, Bates S, Fojo T (2005) A Phase I clinical trial of ixabepilone (BMS-247550), an epothilone B analog, administered intravenously on a daily schedule for 3 days. Cancer 103:1932–1938
Lee JJ, Swain SM (2006) Peripheral neuropathy induced by microtubule-stabilizing agents. J Clin Oncol 24:1633–1642
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
National Cancer Institute Common toxicity criteria v 2.0 (April 30, 1999). Cancer therapy evaluation program. Bethesda, MD, National Cancer Institute
Simon R (1989) Optimal two-stage designs for phase II clinical trials. Control Clin Trials 10:1–10
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
Rights and permissions
About this article
Cite this article
Denduluri, N., Lee, J.J., Walshe, J. et al. Phase II trial of ixabepilone, an epothilone B analog, given daily for three days every three weeks, in metastatic breast cancer. Invest New Drugs 25, 63–67 (2007). https://doi.org/10.1007/s10637-006-9006-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-006-9006-7