Summary
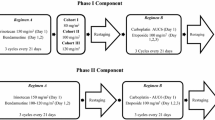
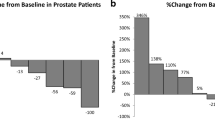
Background This multicenter, open-label, phase Ib study was designed to assess the safety, pharmacokinetics and preliminary efficacy of ME-344, a mitochondrial inhibitor, administered in combination with the topoisomerase I inhibitor, topotecan, in patients with previously treated, locally advanced or metastatic small cell lung (SCLC), ovarian and cervical cancers. Patients and methods In Part 1, patients received ME-344 10 mg/kg intravenously weekly on days 1, 8, 15 and 22 in combination with topotecan 4 mg/m2 on days 1, 8, and 15 of a 28 day cycle. Cycles were repeated until disease progression or unacceptable toxicity. Patients were evaluated for dose-limiting toxicity (DLT) in cycle 1 and ME-344 pharmacokinetic samples were obtained. In Part 2, patients with locally advanced or metastatic SCLC and ovarian cancer were enrolled in expansion cohorts treated at the recommended phase II dose (RP2D) determined in Part 1. Results Fourteen patients were enrolled in Part 1 and no DLTs were observed. The RP2D of ME-344 in combination with topotecan was established as 10 mg/kg. In Part 2, 32 patients were enrolled. The most common treatment-emergent all-grade and grade 3/4 toxicities included fatigue (65.2%, 6.5%), neutropenia (56.5%, 43.5%) and thrombocytopenia (50%, 23.9%). One patient with recurrent ovarian cancer experienced a partial response by RECIST 1.1 and 21 patients achieved stable disease as best response. Conclusions The combination of ME-344 10 mg/kg weekly and topotecan 4 mg/m2 was tolerable, however, the degree of anti-cancer activity does not support further investigation of the combination in unselected patients with SCLC, ovarian and cervical cancers.

Similar content being viewed by others
References
Lopez JS, Banerji U (2017) Combine and conquer: challenges for targeted therapy combinations in early phase trials. Nat Rev Clin Oncol. 14(1):57–66
Igney FH, Krammer PH (2002) Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer 2:277–288
Alvero AB, Montagna MK, Chen R et al (2009) NV-128, a novel isoflavone derivative, induces caspase-independent cell death through the Akt/mammalian target of rapamycin pathway. Cancer 115:3204–3216
Alvero AB, Montagna MK, Holmberg JC et al (2011) Targeting the mitochondria activates two independent cell death pathways in ovarian cancer stem cells. Mol Cancer Ther 10:1385–1393
Lim SC, Carey KT, Mckenzie M (2015) Anti-cancer analogues ME-143 and ME-344 exert toxicity by directly inhibiting mitochondrial NADH: ubiquinone oxidoreductase (complex I). Am J Cancer Res 5:689–701
Jeyaraju DV, Hurren R, Wang X et al (2016) A novel isoflavone, ME-344, targets the cytoskeleton in acute myeloid leukemia. Oncotarget 7:49777–49785
Manevich Y, Reyes L, Britten CD et al (2016) Redox signaling and bioenergetics influence lung cancer cell line sensitivity to the isoflavone ME-344. J Pharmacol Exp Ther 358:199–208
Bendell JC, Patel MR, Infante JR et al (2015) Phase 1, open-label, dose escalation, safety, and pharmacokinetics study of ME-344 as a single agent in patients with refractory solid tumors. Cancer 121:1056–1063
Hirte H, Kennedy EB, Elit L et al (2015) Systemic therapy for recurrent, persistent, or metastatic cervical cancer: a clinical practice guideline. Curr Oncol 22:211–219
Riemsma R, Simons JP, Bashir Z et al (2010) Systematic review of topotecan (Hycamtin) in relapsed small cell lung cancer. BMC Cancer 10:436
Hertzberg RP, Caranfa MJ, Hecht SM (1989) On the mechanism of topoisomerase I inhibition by camptothecin: evidence for binding to an enzyme-DNA complex. Biochemistry 28:4629–4638
Safra T, Berman T, Yachnin A et al (2013) Weekly topotecan for recurrent ovarian, fallopian tube and primary peritoneal carcinoma: tolerability and efficacy study--the Israeli experience. Int J Gynecol Cancer 23:475–480
Sehouli J, Stengel D, Harter P et al (2011) Topotecan weekly versus conventional 5-day schedule in patients with platinum-resistant ovarian cancer: a randomized multicenter phase II trial of the north-eastern German Society of Gynecological Oncology Ovarian Cancer Study Group. J Clin Oncol 29:242–248
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 92:205–216
Dikalova AE, Bikineyeva AT, Budzyn K et al (2010) Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res 107:106–116
Navarro P, Bueno MJ, Zagorac I et al (2016) Targeting tumor mitochondrial metabolism overcomes resistance to Antiangiogenics. Cell Rep 15:2705–2718
Acknowledgements
The authors would like to thank the patients and their families for their participation in this study, as well as the study staff, and the Sarah Cannon Research Institute for managing the study. Martin D. Forster is supported by the UCL/UCLH NIHR Biomedical Research Centre.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was conducted according to ICH guidelines and all sites had IRB/EC approval prior to enrolling patients.
Funding
This study was funded by MEI Pharma, Inc.
Conflict of interest
Jennifer R. Diamond, Barbara Goff, Carolyn D. Britten, Michael S. Gordon, Hani Gabra, David M. Waterhouse, D. Ross Camidge, and Erika P. Hamilton report no potential conflict of interest. Mark Poole is an employee of MEI Pharma. Kathleen M. Moore reports advisory boards for: Astra Zeneca, Advaxis, Clovis, Immunogen, Genentech/Roche and VBL Therapeutics, and Steering Committees for: Advaxis and Astra Zeneca. Johanna C. Bendell reports research funding from Gilead, Genentech, BMS, Five Prime, Lilly, Merck, Medimmune, Celgene, EMD Serono, Taiho, Macrogenetics, GSK, Novartis, OncoMed, LEAP, TG Therapeutics, Astrazeneca, BI, Baiichi Sankyo, Bayer, Incyte, Apexigen, Roche, Koltan, SynDevRx, Forty Seven, Abbvie, StemCentrix, Array, Agios, ARMO, and CytoMx.
Informed consent
All participants gave their informed consent in writing prior to inclusion in the study.
Rights and permissions
About this article
Cite this article
Diamond, J.R., Goff, B., Forster, M.D. et al. Phase Ib study of the mitochondrial inhibitor ME-344 plus topotecan in patients with previously treated, locally advanced or metastatic small cell lung, ovarian and cervical cancers. Invest New Drugs 35, 627–633 (2017). https://doi.org/10.1007/s10637-017-0444-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-017-0444-1