Abstract
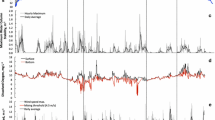
Water samples collected in an acid mine impacted watershed indicated that the concentrations of dissolved trace metals were diurnally influenced by mineral saturation, which is controlled primarily by pH and water temperature. Measurements taken suggested that these variations only occur at sample locations immediately downstream from the confluence of acidic and alkaline waters. It is at these locations where initial mineral precipitation occurred and where subtle changes in solubility were most affected, increasing trace metal removal when both the rate of photosynthesis (influencing pH in headwaters) and water temperature were at a maximum. The role of iron photoreduction (increased midday production of ferrous iron) on overall Cu, Mn, and Zn transport was also evaluated, but found to be inconclusive. Iron photoreduction may however influence adsorption and/or coprecipitation of trace metals through associated changes in oxidation state, solubility, and mineralogy of various iron colloids, which are produced upon the neutralization of acidic, metal enriched water. Furthermore, measured values of copper and zinc were compared to relative USEPA chronic criterion for exposure to continuous concentration (CCC) of metals by the calculation of a “toxicity unit” (TU). It was found that average values of both copper and zinc only exceeded the CCC (TU>1) in the acid mine-impacted Leona Creek. In general, zinc toxicity decreased while copper toxicity increased downstream of the confluence of the mine impacted Leona Creek and background Lion Creek (sampled at Lake Aliso), indicating a significant source of zinc in upstream, non mine-impacted samples.
Similar content being viewed by others
References Cited
Butler TW. 2003 Aqueous Geochemistry of and Acid Mine Impaired Watershed, Oakland, California: California State University, Hayward, University Thesis
California State Mining Bureau, 1902. The Copper Resources of California: Bulletin No. 23. California State Printing Office, Sacramento, pp. 145–146
California Division of Mines and Mining, 1929. Chapter of Report XVII of the State Mineralogist, Mining in California and the activities of the Division of Mines and Mining. California State Printing Office, Sacramento, pp. 440–441
CIMIS. Last Referenced 1/10/2003. California Irrigation Management Information System. California Department of Water Resources. http://www.cimis.water.ca.gov/
Clark CW. 1917 The Geology and ore Deposits of the Leona Rhyolite. University of California Publications. Bulletin of the Department of Geology 10, 361–382
Glover TJ, 1999. Pocket Ref, 2. Sequoia Publishing, Inc. Colorado
Graymer RW. 2000 Geologic map and map database of the Oakland metropolitan area, Alameda, Contra Costa, and San Francisco Counties, California. United States Department of the Interior, United States Geological Survey. Miscellaneous Field Studies MF-2342, Version 1
HACH Company. 1998 Photometric procedures, titration procedures, ion selective titration procedures, microbiological procedures, chemical procedures explained, digesting liquids, oils and solids. HACH Bottled Water Analysis Handbook, 1st Edition. HACH Company
Hem JD, Skougstad MW, 1960. Coprecipitation Effects in Solutions Containing Ferrous, Ferric, and Cupric Ions. Chemistry of Iron in Natural Water. Geological Survey Water-Supply Paper 1459-E. United States Government Printing Office. Washington
Karlsson S, Hakansson K, Ledin A, Allard B, 1995. Light induced changes of Fe(II)/Fe(III) and their implication for colloidal forms of Al, Mn, Cu, Zn, and Cd in an acidic lake polluted with mine waste effluentsJ Geochem Explor 52: 145–159
Kimball BA, Callender E, Axtmann EV, 1995. Effects of colloids on metal transport in a river receiving acid mine drainage, upper Arkansas River, Colorado, USAAppl Geochem 10: 285–306
Krause A, 1928. Transformation of ortho-ferric oxide hydrate into metaferric oxide hydrateZeitscr. Anorg. U. Allg. Chemi. 9: 398–402
Krauskopf KB, Bird DK, 1995. Introduction to Geochemistry, 3. McGraw-Hill, Inc. New York
Liu WX, Coveney RM, Chen JL, 2003. Environmental quality assessment on a river system polluted by mining activitiesAppl Geochem 18: 749–764
McKnight DM, Kimball BA, Runkel RL, 2001. pH dependence of iron photoreduction in a rocky mountain stream affected by acid mine drainageHydrol Processes 15: 1979–1992
Stumm W, Morgan JJ, 1996. Aquatic Chemistry, 3. Wiley Interscience, New York
Tate CM, Broshears RE, McKnight DM, 1995. Phosphate dynamics in an acidic mountain stream: interactions involving algal uptake, sorption of iron oxide, and photoreductionLimnol Oceanogr 40: 939–946
Tessier A, Fortin D, Belzile N, DeVitre RR, Leppard GG, 1996. Metal sorption to diagenetic iron and manganese oxyhydroxides and associated organic matter: Narrowing the gap between field and laboratory measurementsGeochimica et Cosmochimica Acta 60(3): 387–404
USEPA Method 300.0. 1993 Inorganic ions by ion chromatography. United States Environmental Protection Agency. USEPA Report #600/R-93-100
USEPA Method 310.1. 1983 Alkalinity – Titrimetric, pH 4.5. United States Environmental Protection Agency. USEPA Report #600/4-79-020
USEPA Method 6010B. 1996 Inorganics by ICP – Atomic Emission Spectroscopy. United States Environmental Protection Agency. USEPA Report #SW-846 Ch 3.3
USEPA. 2002 National Recommended Water Quality Criteria. United States Environmental Protection Agency. USEPA Report #822-R-02-047
Van den Berg MS, Coops H, Simons J, Pilon J, 2002. A comparative study of the use of inorganic carbon resources by Chara aspera andPotamogeton pectinatus. Aquat Bot 72: 219–233
Webster JG, Swedlund PJ, Webster KS, 1998. Trace metal absorption onto an acid mine drainage iron (III) oxy hydroxy sulfateEnviron Sci Technol 32(10): 1361–1368
Acknowledgements
I would like to thank the staff of the San Francisco Bay Regional Water Quality Control Board for supporting this research; Peter Husby with the United States Environmental Protection Agency, Region 9; and, Dr. Jeff Seitz, Dr.␣Luther Strayer, and Dr. Joy Andrews.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
II, T.W.B. Geochemical and biological controls on trace metal transport in an acid mine impacted watershed. Environ Geochem Health 28, 231–241 (2006). https://doi.org/10.1007/s10653-005-9035-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-005-9035-8