Abstract
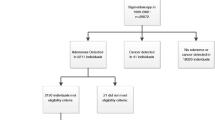
Observational analyses for causal inference often rely on real world data collected for purposes other than research. A frequent goal of these observational analyses is to use the data to emulate a hypothetical randomized experiment, i.e., the target trial, that mimics the design features of a true experiment, including a clear definition of time zero with synchronization of treatment assignment and determination of eligibility. We review a recent observational analysis that explicitly emulated a target trial of screening colonoscopy using insurance claims from U.S. Medicare. We then compare this explicit emulation with alternative, simpler observational analyses that do not synchronize treatment assignment and eligibility determination at time zero and/or do not allow for repeated eligibility. This empirical comparison suggests that lack of an explicit emulation of the target trial leads to biased estimates, and shows that allowing for repeated eligibility increases the statistical efficiency of the estimates.

Similar content being viewed by others
References
Hernán MA, Robins JM. Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol. 2016;183:758–64.
US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW, et al. Screening for colorectal cancer: US preventive services task force recommendation statement. JAMA. 2016;315:2564–75.
Quintero E, Castells A, Bujanda L, Cubiella J, Salas D, Lanas Á, et al. Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med. 2012;366:697–706.
Kaminski MF, Bretthauer M, Zauber AG, Kuipers EJ, Adami H-O, van Ballegooijen M, et al. The NordICC study: rationale and design of a randomized trial on colonoscopy screening for colorectal cancer. Endoscopy. 2012;44:695–702.
Colonoscopy Versus Fecal Immunochemical Test in Reducing Mortality From Colorectal Cancer (CONFIRM) [Internet]. [cited 2015 Jun 3]. https://clinicaltrials.gov/ct2/show/NCT01239082.
Smith RA, Andrews K, Brooks D, DeSantis CE, Fedewa SA, Lortet-Tieulent J, et al. Cancer screening in the United States, 2016: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2016;66:96–114.
García-Albéniz X, Hsu J, Bretthauer M, Hernán MA. Effectiveness of screening colonoscopy to prevent colorectal cancer among medicare beneficiaries aged 70 to 79 years. Ann Intern Med. 2017;166:18.
Stock D, Paszat LF, Rabeneck L. Colorectal cancer mortality reduction is associated with having at least 1 colonoscopy within the previous 10 years among a population-wide cohort of screening age. Gastrointest Endosc. 2016;84:133–41.
Hernán MA, Hernández-Díaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15(5):615–25.
Hernán MA, Sauer BC, Hernández-Díaz S, Platt R, Shrier I. Specifying a target trial prevents immortal time bias and other self-inflicted injuries in observational analyses. J Clin Epidemiol. 2016;79:70–5.
Hernán MA, Alonso A, Logan R, Grodstein F, Michels KB, Willett WC, et al. Observational studies analyzed like randomized experiments: an application to postmenopausal hormone therapy and coronary heart disease. Epidemiology. 2008;19:766–79.
Scholefield JH, Moss SM, Mangham CM, Whynes DK, Hardcastle JD. Nottingham trial of faecal occult blood testing for colorectal cancer: a 20-year follow-up. Gut. 2011;61:1036–40.
Mandel JS, Bond JH, Church TR, Snover DC, Bradley GM, Schuman LM, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota colon cancer control study. N Engl J Med. 1993;328:1365–71.
Acknowledgements
This work was supported by the National Institutes of Health grants K99-CA207730, P01-CA134294, R01-CA164023, R01-HS023128.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10654_2017_287_MOESM1_ESM.pdf
Adjusted cumulative incidence curves under four observational analyses, Medicare 1999–2012. We use t0 to denote time zero of follow-up (PDF 10 kb)
Rights and permissions
About this article
Cite this article
García-Albéniz, X., Hsu, J. & Hernán, M.A. The value of explicitly emulating a target trial when using real world evidence: an application to colorectal cancer screening. Eur J Epidemiol 32, 495–500 (2017). https://doi.org/10.1007/s10654-017-0287-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10654-017-0287-2