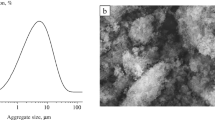
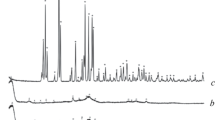
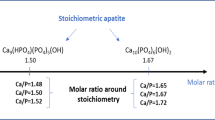
The properties of Ca-deficient hydroxyapatite powder synthesized from calcium nitrate and ammonium hydrophosphate at 60°C, pH = 7, and Ca/P = 1.67, 1.61, and 1.48 are presented. After sintering at 1100°C for 6 h the phase composition of the ceramic based on these powders was represented by tricalcium phosphate (Ca/P = 1.48) or tricalcium phosphate hydroxyapatite (Ca/P = 1.67 and 1.61). The grain size of the ceramic was 100 – 1000 nm.



Similar content being viewed by others
References
M. M. Stevens, “Biomaterials for bone tissue engineering,” Materials Today, 11(5), 18 – 25 (2008).
B. Gossner, “Uber die Kristallstruktur von Glaserit und Kaliumsulfat,” Neues Jb. Miner. Momatsh A, 57, 89 – 116 (1928).
J. H. Welch and W. Gutt, “High temperature studies of the system calcium oxide-phosphorus pentoxide,” J. Chem. Soc., No. 10, 4442 – 4444 (1961).
R. W. Nurse, J. H. Welsch, and W. Gutt, “High temperature equilibria in the system dicalcium silicate – tricalcium phosphate,” Ibid., No. 3, 1077 – 1083 (1959).
K. S. TenHuisen and P. W. Brown, “Phase evolution during the formation of α-tricalcium phosphate,” J. Am. Cer. Soc., 82(10), 2813 – 2818 (1999).
A. Kuznetsov, D. Larioniv, A. Stepuk, et al., “Calcium phosphate scaffolds fabricated via chemical bonding technique from different precursors,” Mat.-wiss. U. Werkstofftech., 40(4), 227 – 284 (2009).
T. V. Safronova, V. I. Putlyaev, M. A. Shekhirev, and A. V. Kuznetsov, “Composite ceramic containing a bioresorbable phase,” Steklo Keram., No. 3, 31 – 35 (2007); T. V. Safronova, V. I. Putlyaev, M. A. Shekhirev, and A. V. Kuznetsov, “Composite ceramic containing a bioresorbable phase,” Glass Ceram., 64(3 – 4), 102 – 106 (2007).
T. V. Safronova, A. V. Kuznetsov, S. A. Korneychuk, et al., “Calcium phosphate powders synthesized from solutions with [Ca2+]/[PO 3-4 ] = 1 for bioresorbable ceramics,” Cent. Eur. J. Chem., 7(2), 184 – 191 (2009).
A. Tas Cuneyt and B. Bhaduri Sarit, “Chemical processing of CaHPO4∙2H2O: its conversion to hydroxyapatite,” J. Am. Ceram. Soc., 87(12), 2195 – 2200 (2004).
M. Tamai, M. Nakamura, T. Isshiki, et al., “Metastable phase in thermal decomposition of Ca-deficient hydroxyapatite,” Mater. Med., 14(7), 617 – 622 (2003).
S. Somrani, C. Rey, and M. Jemal, “Thermal evolution of amorphous tricalcium phosphate,” J. Mater. Chem., 13, 888 – 892 (2003).
E. S. Kovaleva, M. P. Shabanov, V. I. Putlyaev, et al., “Bioresorbable carbonated hydroxyapatite Ca10 – x Na x (PO4)6 – x (CO3) x (OH)2 powders for bioactive materials preparation,” Cent. Eur. J. Chem., 7(2), 168 – 174 (2009).
S. Raynaud, E. Champion, D. Bernache-Assollant, and P. Thomas, “Calcium phosphate apatites with variable Ca/P atomic ratio. I. Synthesis, characterization, and thermal stability of powders,” Biomaterials, 23, 1065 – 1072 (2002).
S. Raynaud, E. Champion, D. Bernache-Assollant, and P. Thomas, “Calcium phosphate apatites with variable Ca/P atomic ratio. II. Calcination and sintering,” Biomaterials, 23, 1073 – 1080 (2002).
M. Villet-Regi, L. M. Rodriguez, and A. J. Salinas, “Synthesis and characterization of calcium deficient apatite,” Solid State Ionics, 101 – 103, 1279 – 1285 (1997).
T. V. Safronova, M. A. Shekhirev, V. I. Putlyaev, and Yu. D. Tret’-yakov, “Ceramic materials based on hydroxylapatite synthesized from solutions with different concentration of the initial reagents,” Inorg. Mater., No. 8, 1005 – 1014 (2007); T. V. Safronova, M. A. Shekhirev, V. I. Putlyaev, and Yu. D. Tret’yakov, “Hydroxyapatite-based ceramic materials prepared using solutions of different concentrations,” Inorg. Mater., 43(8), 901 – 909 (2007).
T. V. Safronova, V. I. Putlyaev,M. A. Shekhirev, and A. V. Kuznetsov, “Disperse systems in the technology of ceramics based on calcium hydroxyapatite,” Steklo Keram., No. 1, 21 – 25 (2007); T. V. Safronova, V. I. Putlyaev, M. A. Shekhirev, and A. V. Kuznetsov, “Disperse systems in calcium hydroxyapatite ceramics technology,” Glass Ceram., 64(1 – 2), 22 – 26 (2007).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Safronova, T.V., Putlyaev, V.I., Avramenko, O.A. et al. Ca-deficient hydroxyapatite powder for producing tricalcium phosphate based ceramics. Glass Ceram 68, 28–32 (2011). https://doi.org/10.1007/s10717-011-9315-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10717-011-9315-y