Abstract
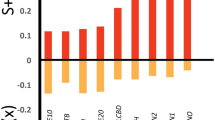
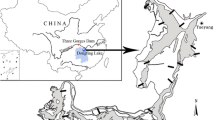
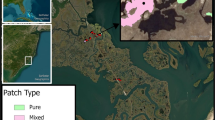
Whether and how the roles of environmental factors in producing vegetation patterns in coastal marshes vary with spatial scale is not well understood. We investigated the relationship between plant communities and edaphic factors in the Yangtze estuary at three spatial scales. Plant communities and edaphic factors were quantified at high and low tidal levels in both freshwater and salt marshes. Canonical correspondence analyses were conducted to examine the relationship between plant communities and edaphic factors at the landscape scale (freshwater vs. salt marsh), the zonation scale (high vs. low tidal level) and the patch scale (dominant vs. other species). Soil salinity, moisture content, pH, bulk density, and organic carbon could well explain segregations of plants at the landscape and zonation scales. However, the same factors exhibited only very weak relationships to plant communities at the patch scale. These results suggest that plant communities in the Yangtze estuary are segregated at different spatial scales by different environmental factors. As spatial scale is often not explicitly addressed investigating community assembly rules, our study underscores the importance of scaling for an improved understanding of community organization in coastal wetlands.






Similar content being viewed by others
References
Adam, P., 1990. Saltmarsh Ecology. Cambridge University Press, Cambridge.
Auestad, I., K. Rydgren & R. H. Okland, 2008. Scale-dependence of vegetation–environment relationships in semi-natural grasslands. Journal of Vegetation Science 19: 139–148.
Baldwin, A. H. & I. A. Mendelssohn, 1998. Response of two oligohaline marsh communities to lethal and nonlethal disturbance. Oecologia 116: 543–555.
Bertness, M. D. & A. M. Ellison, 1987. Determinants of pattern in a New England salt marsh plant community. Ecological Monographs 57: 129–147.
Bertness, M. D. & S. C. Pennings, 2000. Spatial variation in process and pattern in salt marsh plant communities in eastern North America. In Weinstein, M. P. & D. A. Kreeger (eds), Concepts and Controversies in Tidal Marsh Ecology. Kluwer Academic Publishers, New York: 39–57.
Bertness, M. D. & S. D. Hacker, 1994. Physical stress and positive associations among marsh plants. American Naturalist 144: 363–372.
Bertness, M. D., 1991. Zonation of Spartina patens and Spartina alterniflora in New England salt marsh. Ecology 72: 138–148.
Bertness, M. D., L. Gough & S. W. Shumway, 1992. Salt tolerances and the distribution of fugitive salt marsh plants. Ecology 73: 1842–1851.
Crain, C. M., B. R. Silliman, S. L. Bertness & M. D. Bertness, 2004. Physical and biotic drivers of plant distribution across estuarine salinity gradients. Ecology 85: 2539–2549.
Cui, B. S., Q. He & Y. An, 2011. Community structure and abiotic determinants of salt marsh plant zonation vary across topographic gradients. Estuaries and Coasts 34: 459–469.
Emery, N. C., P. J. Ewanchuk & M. D. Bertness, 2001. Competition and saltmarsh plant zonation: stress tolerators may be dominant competitors. Ecology 82: 2471–2485.
Engels, J. G. & K. Jensen, 2010. Role of biotic interactions and physical factors in determining the distribution of marsh species along an estuarine salinity gradient. Oikos 119: 679–685.
Ewanchuk, P. J. & M. D. Bertness, 2004. Structure and organization of a northern New England salt marsh plant community. Journal of Ecology 92: 72–85.
Forey, E., B. Chapelet, Y. Vitasse, M. Tilquin, B. Touzard & R. Michalet, 2008. The relative importance of disturbance and environmental stress at local and regional scales in French coastal sand dunes. Journal of Vegetation Science 19: 493–502.
Gauch, H. G. & R. H. Whittaker, 1981. Hierarchical classification of community data. Journal of Ecology 69: 537–557.
Grace, J. B. & R. G. Wetzel, 1981. Habitat partitioning and competitive displacement in cattails (Typha): experimental field studies. American Natualist 118: 463–474.
Grand, J. & M. J. Mello, 2004. A multi-scale analysis of species–environment relationships: rare moths in a pitch pine–scrub oak (Pinus rigida–Quercus ilicifolia) community. Biological Conservation 119: 495–506.
He, Q., B. S. Cui, Y. Z. Cai, J. F. Deng, T. Sun & Z. F. Yang, 2009. What confines an annual plant to two separate zones along coastal topographic gradients? Hydrobiologia 630: 327–340.
Hubbell, S. P., 2001. The Unified Neutral Theory of Biodiversity and Biogeography. Princeton University Press, Princeton.
Kim, D., D. M. Cairns & J. Bartholdy, 2009. Spatial heterogeneity and domain of scale on the Skallingen salt marsh, Denmark. Danish Journal of Geography 109: 95–112.
Kim, D., D. M. Cairns & J. Bartholdy, 2010. Environmental controls on multiscale spatial pattern of salt marsh vegetation. Physical Geography 31: 58–78.
Kim, D., D. M. Cairns, J. Bartholdy & C. L. S. Morgan, 2012. Scale-dependent correspondence of floristic and edaphic gradients across salt marsh creeks. Annals of the Association of American Geographers 102: 276–294.
Kunza, A. E. & S. C. Pennings, 2008. Patterns of plant diversity in Georgia and Texas salt marshes. Estuaries and Coasts 31: 673–681.
Lepš, J. & P. Šmilauer, 2003. Multivariate Analysis of Ecological Data Using CANOCO. Cambridge University Press, Cambridge.
Levin, S. A., 1992. The problem of pattern and scale in ecology: the Robert H. MacArthur award lecture. Ecology 73: 1943–1967.
Liao, C. Z., Y. Q. Luo, L. F. Jiang, X. H. Zhou, X. W. Wu, C. M. Fang, J. K. Chen & B. Li, 2007. Invasion of Spartina alterniflora enhanced ecosystem carbon and nitrogen stocks in the Yangtze Estuary, China. Ecosystems 10: 1351–1361.
Miller, R. E., J. M. ver Hoef & N. L. Fowler, 1995. Spatial heterogeneity in eight central Texas grasslands. Journal of Ecology 83: 919–928.
Moffett, K. B., D. A. Robinson & S. M. Gorelick, 2010. Relationship of salt marsh vegetation zonation to spatial patterns in soil moisture, salinity and topography. Ecosystems 13: 1287–1302.
Morzaria-Luna, H., J. C. Callaway, G. Sullivan & J. B. Zedler, 2004. Relationship between topographic heterogeneity and vegetation patterns in a Californian salt marsh. Journal of Vegetation Science 14: 523–530.
Münzbergová, Z., 2004. Effect of spatial scale on factors limiting species distributions in dry grassland fragments. Journal of Applied Ecology 92: 854–867.
Noe, G. B. & J. B. Zedler, 2001. Spatio-temporal variation of salt marsh seedling establishment in relation to the abiotic and biotic environment. Journal of Vegetation Science 12: 61–74.
Odum, W. E., 1988. Comparative ecology of tidal freshwater and salt marshes. Annual Review of Ecology and Systematics 19: 147–176.
Palmer, M. W., 1993. Putting things in even better order: the advantages of canonical correspondence analysis. Ecology 74: 2215–2230.
Pennings, S. C. & C. L. Richards, 1998. Effects of wrack burial in salt-stressed habitats: Batis maritima in a southwest Atlantic salt marsh. Ecography 21: 630–638.
Pennings, S. C. & R. M. Callaway, 1992. Salt marsh plant zonation: the relative importance of competition and physical factors. Ecology 73: 681–690.
Pennings, S. C., E. R. Selig, L. T. Houser & M. D. Bertness, 2003. Geographic variation in positive and negative interactions among salt marsh plants. Ecology 84: 1527–1538.
R Development Core Team, 2008. R: A Language and Environment for statistical Computing. R Foundation for Statistical Computing, Vienna.
Reed, R. A., R. K. Peet, M. W. Palmer & P. S. White, 1993. Scale dependence of vegetation-environment correlations: a case study of a North Carolina piedmont woodland. Journal of Vegetation Science 4: 329–340.
Reitalu, T., H. Prentice, M. Sykes, M. Lonn, L. Johansson & K. Hall, 2008. Plant species segregation on different spatial scales in semi-natural grasslands. Journal of Vegetation Science 19: 407–416.
Shipley, B., P. A. Keddy & L. P. Lefkovitch, 1991. Mechanisms producing plant zonation along a water depth gradient: a comparison with the exposure gradient. Canadian Journal of Botany 69: 1420–1424.
Sims, J. R. & V. A. Haby, 1971. Simplified colorimetric determination of soil organic matter. Soil Science 112: 137–141.
Stohlgren, T. J., R. R. Bachand, Y. Onami & D. Binkley, 1998. Species–environment relationships and vegetation patterns: effects of spatial scale and tree life-stage. Plant Ecology 135: 215–228.
ter Braak, C. J. F. & P. Šmilauer, 2002. Canoco for Windows 4.5. Biometris-Plant Research International, Wageningen.
Traut, B. H., 2005. The role of coastal ecotones: a case study of the salt marsh/upland transition zone in California. Journal of Applied Ecology 93: 279–290.
Varty, A. K. & J. B. Zedler, 2008. How waterlogged microsites help an annual plant persist among salt marsh perennials. Estuaries and Coasts 31: 300–312.
Wang, Q., 2007. The dynamics of plant community distribution of the salt marshes in the Yangtze River Estuary as influenced by Spartina alterniflora invasions. PhD Thesis, Fudang University, Shanghai.
Wang, C. H., 2010. Effects of environmental variation on growth, distribution of and interspecific interactions among dominant marsh plants at Chongming Dongtan. PhD Thesis, Fudang University, Shanghai.
Wang, Q., C. H. Wang, B. Zhao, Z. J. Ma, Y. Q. Luo, J. K. Chen & B. Li, 2006. Effects of growing conditions on the growth of and interactions between salt marsh plants: implications for invasibility of habitats. Biological Invasions 8: 1547–1560.
Wang, C., M. Lu, B. Yang, Q. Yang, X. Zhang, T. Hara & B. Li, 2010. Effects of environmental gradients on the performances of four dominant plants in a Chinese saltmarsh: implications for plant zonation. Ecological Research 25: 1–12.
Watson, E. B. & R. Byrne, 2009. Abundance and diversity of tidal marsh plants along the salinity gradient of the San Francisco Estuary: implications for global change ecology. Plant Ecology 205: 113–128.
Weiher, E. & P. A. Keddy, 1995. Assembly rules, null models, and trait dispersion: new questions from old patterns. Oikos 74: 159–164.
Whittaker, R. J. & E. Heegaard, 2003. What is the observed relationship between species richness and productivity? Comment. Ecology 84: 3384–3390.
Whittaker, R. J., K. J. Willis & R. Field, 2001. Scale and species richness: towards a general, hierarchical theory of species diversity. Journal of Biogeography 28: 453–470.
Wiens, J. A., 1989. Spatial scaling in ecology. Functional Ecology 3: 385–397.
Więski, K., H. Guo, C. B. Craft & S. C. Pennings, 2010. Ecosystem functions of tidal fresh, brackish, and salt marshes on the Georgia Coast. Estuaries and Coasts 33: 161–169.
Wilson, S. D. & P. A. Keddy, 1985. Plant zonation on a shoreline gradient: physiological response curves of component species. Journal of Ecology 73: 851–860.
Wilson, S. D. & P. A. Keddy, 1986. Species competitive ability and position along a natural stress/disturbance gradient. Ecology 67: 1236–1242.
Zedler, J. B., J. C. Callaway, J. S. Desmond, G. Vivian-Smith, G. D. Williams, G. Sullivan, A. E. Brewster & B. K. Bradshaw, 1999. Californian salt-marsh vegetation: an improved model of spatial pattern. Ecosystems 2: 19–35.
Acknowledgments
We thank Cheng-Huan Wang and Qiang Yang for field help and discussion, Guo-Xian Song for access to the study sites, and two anonymous reviewers for critical comments. This study was financially supported by National Natural Science Foundation of China (U0833002), National Key Basic Research Program of China (2006CB403303) and China National Funds for Distinguished Young Scientists (51125035).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: K. W. Krauss
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
He, Q., Chen, F., Cui, B. et al. Multi-scale segregations and edaphic determinants of marsh plant communities in a western Pacific estuary. Hydrobiologia 696, 171–183 (2012). https://doi.org/10.1007/s10750-012-1191-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-012-1191-0