Abstract
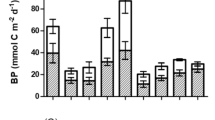
Isoetid macrophytes such as Lobelia dortmanna and Littorella uniflora are engineering species with an extensive root system and high radial oxygen loss. Despite several studies on these macrophytes, the effect of their oxygenation on methane dynamics has never been investigated. In this study, we hypothesise that isoetids promote dissolved inorganic carbon fixation and methane oxidation in sandy sediments. Our whole-ecosystem approach study lasted 2 years (2013–2014) on two oligo-mesotrophic shallow lakes. Benthic chamber incubations confirmed that, as a result of primary production and methanotrophy, isoetid lawns had consistently lower benthic carbon fluxes than bare sediments. On a daily basis, vegetated areas acted as a carbon sink (−0.7 ± 0.4 g C m−2 days−1, as DIC + CH4), whereas bare sediments acted as a net source (0.6 ± 0.5 g C m−2 days−1, as DIC + CH4). Photosynthetic quotients of <1 indicated that photosynthetically produced oxygen was not released into the water column, but accumulated in leaf lacunae or was transferred to the rhizosphere, that contributing to the alteration of net benthic fluxes at the sediment–water interface. This preliminary study highlights the necessity of further investigating the role that isoetids play in mitigating greenhouse gas emissions from temperate shallow lakes.




Similar content being viewed by others
References
Anderson, L. G., P. O. J. Hall, Å. Iverfeldt, B. van der Loeff, B. Sundby & S. F. G. Westerlund, 1986. Benthic respiration measured by total carbonate production. Limnology and Oceanography 31: 319–329.
Arts, G. H. P., 2002. Deterioration of atlantic soft water macrophyte communities by acidification, eutrophication and alkalinisation. Aquatic Botany 73: 373–393.
Attermeyer, K., S. Flury, R. Jayakumar, P. Fiener, K. Steger, V. Arya & K. Premke, 2016. Invasive floating macrophytes reduce greenhouse gas emissions from a small tropical lake. Scientific Reports 6: 20424.
Baastrup-Spohr, L., C. L. Møller & K. Sand-Jensen, 2016. Water-level fluctuations affect sediment properties, carbon flux and growth of the isoetid Littorella uniflora in oligotrophic lakes. Freshwater Biology 61: 301–315.
Bartoli, M., D. Nizzoli & P. Viaroli, 2003. Microphytobenthos activity and fluxes at the sediment-water interface: interactions and spatial variability. Aquatic Ecology 37: 341–349.
Bastviken, D., J. Cole, M. Pace & L. Tranvik, 2004. Methane emissions from lakes: dependence of lake characteristics, two regional assessments, and a global estimate. Global Biogeochemical Cycles 18(4): 1–12.
Bastviken, D., L. J. Tranvik, J. A. Downing, P. M. Crill & A. Enrich-Prast, 2011. Freshwater methane emissions offset the continental carbon sink. Science 331: 50.
Bertrin, V., S. Boutry, A. Dutartre & E. Lambert, 2013. Communautés de Characées des lacs médocains (Sud-Ouest de la France). Eléments d’écologie et de distribution. Acta Botanica Gallica: Botany Letters 160: 131–140.
Bolpagni, R., E. Pierobon, D. Longhi, D. Nizzoli, M. Bartoli, M. Tomaselli & P. Viaroli, 2007. Diurnal exchanges of CO2 and CH4 across the water-atmosphere interface in a water chestnut meadow (Trapa natans L.). Aquatic Botany 87: 43–48.
Boon, P. I. & B. K. Sorrell, 1991. Biogeochemistry of billabong sediments. The effect of macrophytes. Freshwater Biology 26: 209–226.
Boston, H. L. & M. S. Adams, 2007. Productivity, growth and photosynthesis of two small isoetid plants Littorella uniflora and Isoetes macrospora. Journal of Ecology 75: 333–350.
Canfield, D.E., B.B. Jørgensen, H. Fossing, R. Glud, J. Gundersen, N.B. Ramsing …& P.O. Hall, 1993. Pathways of organic carbon oxidation in three continental margin sediments. Marine Geology 113: 27–40
Caraco, N. F. & J. J. Cole, 2002. Contrasting impacts of a native and alien macrophyte on dissolved oxygen in a large river. Ecological Applications 5: 1496–1509.
Cellamare, M., S. Morin, M. Coste & J. Haury, 2012. Ecological assessment of French Atlantic lakes based on phytoplankton, phytobenthos and macrophytes. Environmental Monitoring and Assessment 184: 4685–4708.
Clément, B. & A. Aidoud, 2009. Resistance against eutrophication based on 40-year diachronic study (1966–2006) on marginal wetlands of oligotrophic shallow lakes in south-west of France. Rapport du projet européen Eurolimpacs 2009, 27 pp.
Dean Jr., W. E., 1974. Determination of carbonate and organic matter in calcareous sediments and sedimentary rocks by loss on ignition: comparison with other methods. Journal of Sedimentary Research 44(1): 242–248.
den Heyer, C. & J. Kalff, 1998. Organic matter mineralization rates in sediments: a within- and among-lake study. Limnology and Oceanography 43: 695–705.
Ding, W., Z. Cai & H. Tsuruta, 2005. Plant species effects on methane emissions from freshwater marshes. Atmospheric Environment 39: 3199–3207.
Duan, X., X. Wang, Y. Mu & Z. Ouyang, 2005. Seasonal and diurnal variations in methane emissions from Wuliangsu Lake in arid regions of China. Atmospheric Environment 39(25): 4479–4487.
Dutartre, A., 1984. Données préliminaires sur les macrophytes immergées du lac de Biscarosse-Cazaux-Sanguinet (Aquitaine). Revue française des sciences de l’eau 3: 409–419.
Farmer, A. M. & D. H. N. Spence, 1987. Environmental control of the seasonal growth of the submersed aquatic macrophyte Lobelia dortmanna L. New Phytologist 106: 289–299.
Froelich, P.N., G.P. Klinkhammer, M.A.A. Bender, N.A. Luedtke, G.R. Heath, D. Cullen … & V. Maynard, 1979. Early oxidation of organic matter in pelagic sediments of the eastern equatorial Atlantic: suboxic diagenesis. Geochimica et Cosmochimica Acta 43: 1075–1090.
Heilman, M. & R. G. Carlton, 2001. Methane oxidation associated with submerged vascular macrophytes and its impact on plant diffusive methane flux. Biogeochemistry 52: 207–224.
Hirota, M., Y. Tang, Q. Hu, S. Hirata, T. Katoa, W. Mo, G. Cao & S. Mariko, 2004. Methane emissions from different vegetation zones in a Qinghai-Tibetan Plateau wetland. Soil Biology & Biochemistry 36: 737–748.
Hunding, C., 1973. Diel variation in oxygen production and uptake in a microbenthic littoral community of a nutrient-poor lake. Oikos 24: 352–360.
Kufel, L., E. Biardzka & M. Strzałek, 2013. Calcium carbonate incrustation and phosphorus fractions in five charophyte species. Aquatic Botany 109: 54–57.
Lorenzen, C. J., 1967. Determination of chlorophyll and phaeopigments: spectrophotometric equations. Limnology and Oceanography 12: 343–346.
Madsen, T. V., 1987. Interactions between internal and external CO2 pools in the photosynthesis of the aquatic plants Littorella uniflora (L.) Aschers and Isoetes lacustris L. New Phytologist 106: 35–50.
McAuliffe, C., 1971. GC determination of solutes by multiple phase equilibrium. Chemical Technology 1: 46–51.
Møller, C. L. & K. Sand-Jensen, 2012. Rapid oxygen exchange across the leaves of Littorella uniflora provides tolerance to sediment anoxia. Freshwater Biology 57: 1875–1883.
Nizzoli, D., E. Carraro, V. Nigro & P. Viaroli, 2010. Effect of organic enrichment and thermal regime on denitrification and dissimilatory nitrate reduction to ammonium (DNRA) in hypolimnetic sediments of two lowland lakes. Water Research 44: 2715–2724.
Pedersen, O., C. Pulido, S. M. Rich & T. D. Colmer, 2011. In situ O2 dynamics in submerged Isoetes australis: varied leaf gas permeability influences underwater photosynthesis and internal O2. Journal of Experimental Botany 62: 4691–4700.
Peixoto, R. B., H. Marotta, D. Bastviken & A. Enrich-Prast, 2016. Floating aquatic macrophytes can substantially offset open water CO2 emissions from tropical floodplain lake ecosystems. Ecosystems 19: 724–736.
Pinardi, M., M. Bartoli, D. Longhi, U. Marzocchi, A. Laini, C. Ribaudo & P. Viaroli, 2009. Benthic metabolism and denitrification rates in a river reach: a comparison between vegetated and bare sediments. Journal of Limnology 68: 133–145.
Pukacz, A., M. Pelechaty & M. Frankowski, 2014. Carbon dynamics in a hardwater lake: effect of charophyte biomass on carbonate deposition. Polish Journal of Ecology 62(4): 695–705.
Pulido, C., E. Lucassen, O. Pedersen & M. Roelofs, 2010. Influence of quantity and lability of sediment organic matter on the biomass of two isoetids, Littorella uniflora and Echinodorus repens. Freshwater Biology 56: 939–951.
Racchetti, E., M. Bartoli, C. Ribaudo, D. Longhi, E. Q. L. Brito, C. Naldi, P. Iacumin & P. Viaroli, 2010. Short term changes in pore water chemistry in river sediments during the early colonization by Vallisneria spiralis. Hydrobiologia 652: 127–137.
Ribaudo, C., M. Bartoli, E. Racchetti, D. Longhi & P. Viaroli, 2011. Seasonal fluxes of O2, DIC and CH4 in sediments with Vallisneria spiralis: indications for radial oxygen loss. Aquatic Botany 94: 134–142.
Ribaudo, C., M. Bartoli, D. Longhi, S. Castaldi, S. C. Neubauer & P. Viaroli, 2012. CO2 and CH4 fluxes across a Nuphar lutea (L.) Sm. stand. Journal of Limnology 71: 200–210.
Ribaudo, C., V. Bertrin & A. Dutartre, 2014. Dissolved gas and nutrient dynamics within an Egeria densa Planch. bed. Acta Botanica Gallica: Botany Letters 161: 233–241.
Rich, P. H. & R. G. Wetzel, 1978. Detritus in the lake ecosystem. American Naturalist 112: 57–71.
Richardson, K., H. Griffiths, M. L. Reed, J. A. Raven & N. M. Griffiths, 1984. Inorganic carbon assimilation in the Isoetids, Isoetes lacustris L. and Lobelia dortmanna L. Oecologia 61: 115–121.
Risgaard-Petersen, N. & K. Jensen, 1997. Nitrification and denitrification in the rhizosphere of the aquatic macrophyte Lobelia dortmanna L. Limnology and Oceanography 42: 529–537.
Roelofs, J. G. M., J. A. A. R. Schuurkes & A. J. M. Smits, 1984. Impact of acidification and eutrophication on macrophyte communities in soft waters. II. Experimental studies. Aquatic Botany 18: 389–411.
Sand-Jensen, K. & M. Søndergaard, 1979. Distribution and quantitative development of aquatic macrophytes in relation to sediment characteristics in oligotrophic Lake Kalgaard, Denmark. Freshwater Biology 9: 1–11.
Sand-Jensen, K. & C. Prahl, 1982. Oxygen exchange with the lacunae and across the leaves and roots of the submerged vascular macrophyte Lobelia dortmanna L. New Phytologist 91: 103–120.
Schuurkes, J. A. A. R., C. J. Kok & C. den Hartog, 1986. Ammonium and nitrate uptake by aquatic plants from poorly buffered and acidified waters. Aquatic Botany 24: 131–146.
Siong, K. & T. Asaeda, 2009. Effect of magnesium on charophytes calcification: implications for phosphorus speciation stored in biomass and sediment in Myall Lake (Australia). Hydrobiologia 632: 247–259.
Soana, E., M. Naldi, S. Bonaglia, E. Racchetti, G. Castaldelli, V. Brüchert, P. Viaroli & M. Bartoli, 2015. Benthic nitrogen metabolism in a macrophyte meadow (Vallisneria spiralis L.) under increasing sedimentary organic matter loads. Biogeochemistry 124: 387–404.
Smolders, A. J. P., E. Lucassen & J. G. M. Roelofs, 2002. The isoetid environment: biogeochemistry and threats. Aquatic Botany 73: 325–350.
Søndergaard, M. & K. Sand-Jensen, 1979. Carbon uptake by leaves and roots of Littorella uniflora (L.) Aschers. Aquatic Botany 6: 1–12.
Sorrell, B. K., M. T. Downes & C. L. Stanger, 2002. Methanotrophic bacteria and their activity on submerged aquatic macrophytes. Aquatic Botany 72: 107–119.
St.Louis, V. L., C. A. Kelly, É. Duchemin, J. W. M. Rudd & D. M. Rosenberg, 2000. Reservoir surfaces as sources of greenhouse gases to the atmosphere: a global estimate. Bioscience 50: 766–775.
Sweerts, J. P. R., M. J. Baer-Gilissen, A. A. Cornelese & T. E. Cappenberg, 1991. Oxygen-consuming processes at the profundal and littoral sediment-water interface of a small meso-eutrophic lake (Lake Vechten, The Netherlands). Limnology and Oceanography 36(6): 1124–1133.
Tastet, J.-P., R. Lalanne, B. Maurin & B. Dubos, 2008. Geological and archaeological chronology of a late Holocene coastal enclosure: The Sanguinet lake (SW France). Geoarchaeology 23: 131–149.
Vanden Berghen, C., 1964. La végétation des rives du lac de Hourtin (Gironde, France). Bulletin du Jardin Botanique de l’Etat à Bruxelles 34: 243–267.
van der Nat, F.-J. W. A. & J. J. Middelburg, 1998. Seasonal variation in methane oxidation by the rhizosphere of Phragmites australis and Scirpus lacustris. Aquatic Botany 61: 95–110.
van Luijn, F., P. C. M. Boers, L. Lijklema & J.-P. R. A. Sweerts, 1999. Nitrogen fluxes and processes in sandy and muddy sediments from a shallow eutrophic lake. Water Research 33: 33–42.
Velasco, J., A. Millan, M. R. Vidal-Abarca, M. L. Suarez, C. Guerrero & M. Ortega, 2003. Macrophytic, epipelic and epilithic primary production in a semiarid Mediterranean stream. Freshwater Biology 48: 1408–1420.
Weiss, R. F., 1970. The solubility of nitrogen, oxygen and argon in water and seawater. Deep Sea Research and Oceanographic Abstracts 17: 721–735.
Winkler, L., 1888. Die Bestimmung des in Wasser Gelösten Sauerstoffes. Ber Dtsch Chem Ges 21: 2843–2855.
Wium-Andersen, S., 1971. Photosynthetic uptake of free CO2 by the roots of Lobelia dortmanna. Physiologia Plantarum 25: 245–248.
Yamamoto, S., J. B. Alcauskas & T. E. Crozier, 1976. Solubility of methane in distilled water and seawater. Journal of Chemical Engineering Data 21: 78–80.
Yoshida, N., H. Iguchi, H. Yurimoto, A. Murakami & Y. Sakai, 2014. Aquatic plant surface as a niche for methanotrophs. Frontiers in Microbiology 5: 30.
Yvon-Durocher, G., J. M. Montoya, G. Woodward, J. J. Jones & M. Trimmer, 2011. Warming increases the proportion of primary production emitted as methane from freshwater mesocosms. Global Change Biology 17: 1225–1234.
Zilius, M., D. Daunys, J. Petkuviene & M. Bartoli, 2012. Sediment-water oxygen, ammonium and soluble reactive phosphorus fluxes in a turbid freshwater estuary (Curonian lagoon, Lithuania): evidences of benthic microalgal activity. Journal of Limnology 714: 309–319.
Zilius, M., M. Bartoli, M. Bresciani, M. Katarzyte, T. Rignis, J. Petkuviene, I. Lubiene, C. Giardino, P. A. Bukaveckas, R. de Wit & A. Razinkovas-Baziukas, 2014. Feedback mechanisms between cyanobacterial blooms, transient hypoxia, and benthic phosphorus regeneration in shallow coastal environments. Estuaries and Coasts 37: 680–694.
Acknowledgments
Authors wish to thank K. Madarassou and M. Eon for nutrients analyses. T. Huguet, S. Moreira, G. Ducasse, J. Chabanne, J-C. Gregoire and D. Poirier also participated to field and laboratory activities. M. Bartoli (University of Parma, Italy) and A. Dutartre (Agence Régionale pour la Biodiversité - Aquitaine) provided technical and experiential expert advice. This work was funded by AEAG (Agence de l’Eau Adour-Garonne), within the conventions #310330085 and #310330109, by Irstea (Institut de Recherche Sciences et Technologies pour l’Environnement et l’Agriculture) and by Université de Bordeaux - CNRS UMR 5805.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Sidinei Magela Thomaz
Rights and permissions
About this article
Cite this article
Ribaudo, C., Bertrin, V., Jan, G. et al. Benthic production, respiration and methane oxidation in Lobelia dortmanna lawns. Hydrobiologia 784, 21–34 (2017). https://doi.org/10.1007/s10750-016-2848-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-016-2848-x