Abstract
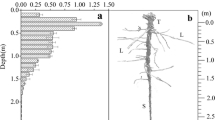
Mangrove trees are rooted in saline soils which can limit their growth. Access to alternative, less saline water sources may provide important water subsidies. We assessed the hydrogen and oxygen isotopic signatures of soil porewater, groundwater and atmospheric water sources (dew and rainfall) and tree stem water from three mangroves species over two sites that varied in elevation. Although stem water isotopic signatures were most similar to porewater, variation in isotopic values indicated trees also accessed alternative water sources, the degree to which varied over sites and among species. Rhizophora stylosa had lowest values of stable isotopes among the species indicating significant groundwater utilization. In a long-term fertilization experiment we found that growth of Ceriops australis and Lumnitzera racemosa was nitrogen limited, while growth of R. stylosa was nitrogen limited to a lesser extent, suggesting groundwater may also provide nutritional benefits for R. stylosa. The uptake of alternative water sources in addition to saline porewater may improve metabolic function, differentially altering the performance of different species and over sites.




Similar content being viewed by others
References
Ball, M. C., 1988. Ecophysiology of mangroves. Trees 2:129–142.
Ball, M. C., I. R. Cowan & G. D. Farquhar, 1988. Maintenance of leaf temperature and the optimisation of carbon gain in relation to water loss in a tropical mangrove forest. Functional Plant Biology 15: 263–276.
Burgess, S. S. O. & T. E. Dawson, 2004. The contribution of fog to the water relations of Sequoia sempervirens (D. Don): foliar uptake and prevention of dehydration. Plant, Cell and Environment 27: 1023–1034.
Bratton, J. F., J. K. Böhlke, D. E. Krantz & C. R. Tobias, 2009. Flow and geochemistry of groundwater beneath a back-barrier lagoon: The subterranean estuary at Chincoteague Bay, Maryland, USA. Marine Chemistry 113: 78–92.
Casper, B. B. & R. B. Jackson, 1997. Plant competition underground. Annual Reviews in Ecology and Systematics 28: 545–570.
Castaneda-Moya, E., R. R. Twilley, V. H. Rivera-Monroy, B. D. Marx, C. Coronado-Molina & S. M. L. Ewe, 2011. Patterns of root dynamics in mangrove forests along environmental gradients in the Florida Coastal Everglades, USA. Ecosystems 14: 1178–1195.
Davies, P. L. & B. D. Eyre, 2005. Estuarine modification of nutrient and sediment exports to the Great Barrier Reef Marine Park from the Daintree and Annan River catchments. Marine Pollution Bulletin 51: 174–185.
Dawson, T. E. & J. R. Ehleringer, 1993. Isotopic enrichment of water in the “woody” tissues of plants: implications for plant water source, water uptake, and other studies which use the stable isotopic composition of cellulose. Geochimica et Cosmochimica Acta 57: 3487–3492.
Duke, N., M. C. Ball & J. Ellison, 1998. Factors influencing biodiversity and distributional gradients in mangroves. Global Ecology and Biogeography Letters 7: 27–47.
Ehleringer, J. & T. Dawson, 1992. Water uptake by plants: perspectives from stable isotope composition. Plant Cell and Environment 15: 1073–1082.
Eichert, T., A. Kurtz, U. Steiner & H. E. Goldbach, 2008. Size exclusion limits and lateral heterogeneity of the stomatal foliar uptake pathway for aqueous solutes and water-suspended nanoparticles. Physiologia Plantarum 134: 151–160.
Eller, C. B., A. L. Lima & R. S. Oliveira, 2013. Foliar uptake of fog water and transport belowground alleviates drought effects in the cloud forest tree species, Drimys brasiliensis (Winteraceae). New Phytologist 199: 151–162.
Ellsworth, P. & D. Williams, 2007. Hydrogen isotope fractionation during water uptake by woody xerophytes. Plant and Soil 291:93–107.
Ewe, S., L. S. L. Sternberg & D. Childers, 2007. Seasonal plant water uptake patterns in the saline southeast Everglades ecotone. Oecologia 152:607–616.
Fargione, J., & D. Tilman, 2005. Niche differences in phenology and rooting depth promote coexistence with a dominant C4 bunchgrass. Oecologia 143(4): 598–606.
Fass, T., P. Cook, T. Stieglitz & A. Herczeg, 2007. Development of saline ground water through transpiration of sea water. Groundwater. doi:10.1111/j.1745-658.4.2007.00344.x.
February, E. C., N. Allsopp, T. Shabane & D. Hattas, 2011. Coexistence of a C4 grass and a leaf succulent shrub in an arid ecosystem. The relationship between rooting depth, water and nitrogen. Plant and Soil 349: 253–260.
Feller, I. C., 1995. Effects of nutrient enrichment on growth and herbivory of dwarf red mangrove (R. stylosa mangle). Ecological Monographs 65: 477–505.
Feller, I. C., K. L. McKee, D. F. Whigham & J. P. O’Neill, 2003. Nitrogen vs. phosphorus limitation across an ecotonal gradient in a mangrove forest. Biogeochemistry 62: 145–175.
Greaver, T. L. & L. S. L. Sternberg, 2006. Linking marine resources to ecotonal shifts of water uptake by terrestrial dune vegetation. Ecology 87: 2389–2396.
Groom, P. K., 2004. Rooting depth and plant water relations explain species distribution patterns within a sandplain landscape. Functional Plant Biology 31(5): 423–428.
Johnes, P. J. & A. L. Heathwaite, 1992. A procedure for the simultaneous determination of total nitrogen and total phosphorus in freshwater samples using persulphate microwave digestion. Water Research 26: 1281–1287.
Johnson, D. M. & W. K. Smith, 2008. Cloud immersion alters microclimate, photosynthesis and water relations in Rhododendron catawbiense and Abies fraseri seedlings in the southern Appalachian Mountains, USA. Tree Physiology 28: 385–392.
Lambs, L., E. Muller & F. Fromard, 2008. Mangrove trees growing in a very saline condition but not using seawater. Rapid Communications in Mass Spectrometry 22: 2835–2843.
Lambs, L., P. Mangion, E. Mougin & F. Fromard, 2016. Water cycle and salinity dynamics in the mangrove forests of Europa and Juan de Nova Islands, southwest Indian Ocean. Rapid Communications in Mass Spectrometry 30: 311–320.
Larsen, G. R. & M. E. Cox, 2011. Hydrochemical and isotopic characterisation of groundwaters to define aquifer type and connectivity in a subtropical coastal setting, Fraser Coast, Queensland. Environmental and Earth Sciences 64: 1885–1909.
Lin, G. & L. S. L. Sternberg, 1993. Hydrogen isotopic fractionation by plant roots during water uptake in coastal wetland plants In Ehleringer, J. R., A. E. Hall, G. D. Farquhar (eds), Stable Isotopes and Plant Carbon-Water Relations. Academic Press Inc., New York.
Liu, J., G. Fu, X. Song, S. P. Charles, Y. Zhang, D. Han & S. Wang, 2010. Stable isotopic compositions in Australian precipitation. Journal of Geophysical Research 115: D23307.
Maher, D. T., I. R. Santos, L. Golsby-Smith, J. Gleeson & B. D. Eyre, 2013. Groundwater-derived dissolved inorganic and organic carbon exports from a mangrove tidal creek: the missing mangrove carbon sink? Limnology and Oceanography 58: 475–488.
Martin, C. E. & D. J. von Willert, 2000. Leaf epidermal hydathodes and the ecophysiological consequences of foliar water uptake in species of Crassula from the Namib Desert in southern Africa. Plant Biology 2: 229–242.
McKee, K. L., 1996. Growth and physiological responses of neotropical mangrove seedlings to root zone hypoxia. Tree Physiology 16: 883–889.
McKee, K. L., I. A. Mendelssohn & M. W. Hester, 1988. Reexamination of pore water sulfide concentrations and redox potentials near the aerial roots of R. stylosa mangle and Avicennia germinans. American Journal of Botany 75:1352–1359.
McKee, K. L., I. C. Feller, M. Popp & W. Wanek, 2002. Mangrove isotopic (δ15N and δ13C) fractionation across a nitrogen vs. phosphorus limitation gradient. Ecology 83: 1065–1075.
Michael, H. A., A. E. Mulligan & C. F. Harvey, 2005. Seasonal oscillations in water exchange between aquifers and the coastal ocean. Nature 436: 1145–1148.
Moon, G., B. Clough, C. Peterson & W. Allaway, 1986. Apoplastic and symplastic pathways in Avicennia marina (Forsk.) Vierh. Roots revealed by fluorescent tracer dyes. Functional Plant Biology 13: 637–648.
Munné-Bosch, S. & L. Alegre, 1999. Role of dew on the recovery of water-stressed Melissa officinalis L. plants. Journal of Plant Physiology 154: 759–766.
Paul, D., G. Skrzypek & I. Forizs, 2007. Normalization of measured stable isotope composition to isotope reference scale – a review. Rapid Communications in Mass Spectrometry 21: 3006–3014.
Phillips, D. L. & J. W. Gregg, 2001. Uncertainty in source partitioning using stable isotopes. Oecologia 127: 171–179. See also erratum – Oecologia 128: 304.
R Development Core Team, 2008. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org.
Reef, R. & C. E. Lovelock, 2014. Regulation of water balance in mangroves. Annals of Botany. doi:10.1093/aob/mcu174.
Reef, R., I. C. Feller & C. E. Lovelock, 2010. Nutrition of mangroves. Tree Physiology 30: 1148–1160.
Reef, R., H. L. Markham, N. S. Santini & C. E. Lovelock, 2015. The response of the mangrove Avicennia marina to heterogeneous salinity measured using a split-root approach. Plant and Soil 393: 297–305.
Saha, A. K., L. S. L. Sternberg, M. S. Ross & F. Miralles-Wilhelm, 2010. Water source utilization and foliar nutrient status differs between upland and flooded plant communities in wetland tree islands. Wetland Ecology and Management 18: 343–355.
Santos, I. R., B. D. Eyre & M. Huettel, 2012. The driving forces of porewater and groundwater flow in permeable coastal sediments: a review. Estuarine and Coastal Shelf Science 98: 1–15.
Schulze, E. D., H. A. Mooney, O. E. Sala, E. Jobbagy, N. Buchmann, G. Bauer, J. Canadell, R. B. Jackson, J. Loreti, M. Oesterheld & J. R. Ehleringer, 1996. Rooting depth, water availability, and vegetation cover along an aridity gradient in Patagonia. Oecologia 108(3): 503–511.
Semeniuk, V., 1983. Mangrove distribution in Northwestern Australia in relationship to regional and local freshwater seepage. Vegetatio 53: 11–31.
Silvertown J. 2004. Plant coexistence and the niche. Trends in Ecology & Evolution 19: 605–611.
Slayter, R. O., 1956. Absorption of water from atmospheres of different humidity and its transport through plants. Australian Journal of Biological Science 9: 552–558.
Sternberg, L. S. L. & P. K. Swart, 1987. Utilization of freshwater and ocean water by coastal plants of Southern Florida. Ecology 68:1898–1905.
Stieglitz, T., 2005. Submarine groundwater discharge into the near-shore zone of the Great Barrier Reef, Australia. Marine Pollution Bulletin 51: 51–59.
Tamooh, F., M. Huxham, M. Karachi, M. Mencuccini, J. Kairo & B. Kirui, 2008. Below-ground root yield and distribution in natural and replanted mangrove forests at Gazi bay, Kenya. Forest Ecology and Management 256: 1290–1297.
Tomlinson, P. B., 1987. The Botany of Mangroves. Cambridge University Press, Cambridge.
Valiela, I., J. Costa, K. Foreman, J. M. Teal, B. Howes & D. Aubrey, 1990. Transport of groundwater-borne nutrients from watersheds and their effects on coastal waters. Biogeochemistry 10: 177–197.
Wei, L., D. A. Lockington, S. C. Poh, M. Gasparon & C. E. Lovelock, 2012. Water use patterns of estuarine vegetation in a tidal creek system. Oecologia 172: 485–494.
West, A. G., S. J. Patrickson & J. R. Ehleringer, 2006. Water extraction times for plant and soil materials used in stable isotope analysis. Rapid Communication in Mass Spectrometry 20:1317–1321.
Yakir, D. & Y. Yechielie, 1995. Plant invasion of newly exposed hypersaline Dead Sea shores. Nature 374: 803–805.
Zhai, L., J. Jiang, D. DeAngelis & L. S. L. Sternberg, 2016. Prediction of plant vulnerability to salinity increase in a coastal ecosystem by stable isotope composition (δ18O) of plant stem water: a model study. Ecosystems 19(1): 32–49.
Acknowledgements
We thank Catherine Bone and Nigel Brothers for their support during field campaigns. This work was supported by the Australian Research Council (ARC) Centre for Excellence for Groundwater Research and Training, ARC Discovery Projects DP1096749 and DP150104437 awarded to CEL and MCB, and DE120101706 awarded to RR.
Author contribution
All authors conceived, designed and performed the experiments. CEL and RR analysed the data. CEL wrote the manuscript; RR and MCB edited the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: K. W. Krauss, I. C. Feller, D. A. Friess & R. R. Lewis III / Causes and Consequences of Mangrove Ecosystem Responses to an Ever-Changing Climate
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lovelock, C.E., Reef, R. & Ball, M.C. Isotopic signatures of stem water reveal differences in water sources accessed by mangrove tree species. Hydrobiologia 803, 133–145 (2017). https://doi.org/10.1007/s10750-017-3149-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-017-3149-8