Abstract
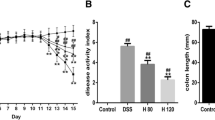
The aim of this study was to investigate the effect of luteolin (Lu) on dextran sodium sulfate (DSS)–induced colitis in mice. Mice spleen was weighed. The length of colon was measured. H&E staining was used to observe the pathological changes of colon in mice. Superoxide dismutase (SOD) and malondialdehyde (MDA) in serum and intestine of mice were detected by commercial kits. Serum and intestinal cytokines were detected by ELISA kits. The expression of HMGB1 mRNA was detected by real-time PCR. The expression of HMGB1-TLR-NF-κB pathway was detected by Western blot. The level of HMGB1 was detected by immunohistochemistry. The results showed that Lu significantly increased the colon length/body weight ratio and significantly decreased the spleen weight/body weight ratio. Lu significantly increased serum and intestinal SOD levels and decreased MDA levels. Lu significantly increased serum and intestinal cytokine levels in mice. qPCR and immunohistochemistry results showed that Lu significantly reduced HMGB1 mRNA level and protein level. In addition, Lu significantly reduced the expression of HMGB1-TLR-NF-κB signaling pathway protein of intestine in mice. In conclusion, Lu significantly reduced and alleviated DSS-induced colitis in mice, and the mechanism was related to the regulation of intestinal HMGB1-TLR-NF-κB signaling pathway in mice.









Similar content being viewed by others
References
Bannwart, C.F., E. Nakaira-Takahagi, M.A. Golim, et al. 2010. Downregulation of nuclear factor-kappa B (NF-kappa B) pathway by silibinin in human monocytes challenged with Paracoccidioides brasiliensis [J]. Life Sciences 86 (23–24): 880–886.
Kaser, A., S. Zeissig, and R.S. Blumberg. 2010. Inflammatory bowel disease[J]. Annual Review of Immunology 28: 573–621.
Jess, T., C. Rungoe, and L. Peyrin-Biroulet. 2012. Risk of colorectal cancer in patients with ulcerative colitis: a meta-analysis of population-based cohort studies [J]. Clin Gastroenterol Hepatol 10 (6): 639–645.
Ng, S.C., C.N. Bernstein, M.H. Vatn, et al. 2013. Geographical variability and environmental risk factors in inflammatory bowel disease [J]. Gut 62 (4): 630–649.
Molodecky, N.A., I.S. Soon, D.M. Rabi, et al. 2012. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review [J]. Gastroenterology 142 (1): 46–54.
Lingna, Y., C. Qian, and C. Jianfeng. 2013. Review of inflammatory bowel disease in China. The Scientific World Journal 2013: 296470.
Yang, S.K., S. Yun, J.H. Kim, et al. 2008. Epidemiology of inflammatory bowel disease in the Songpa-Kangdong district, Seoul, Korea, 1986–2005: a KASID study [J]. Inflammatory Bowel Diseases 124 (4): A210.
Thia, K.T., Edward V. Loftus Jr., W.J. Sandborn, et al. 2008. An update on the epidemiology of inflammatory bowel disease in Asia[J]. American Journal of Gastroenterology 103 (12): 3167.
Bianchi, M.E., and A.A. Manfredi. 2007. High-mobility group box 1(HMGB1) protein at the crossroads between innate and adaptive immunity[J]. Immunological Reviews 220 (1): 35–46.
Wirtz, S., and M.F. Neurath. 2000. Animal models of intestinal inflammation: new insights into the molecular pathogenesis and immunotherapy of inflammatory bowel disease [J]. International Journal of Colorectal Disease 15 (3): 144–160.
Stros, M. 2010. HMGB proteins: interactions with DNA and chromatin. Biochim Biophys Acta 1799: 101–113.
Bustin, M. 1999. Regulation of DNA-dependent activities by the functional motifs of the high-mobility-group chromosomal proteins. Molecular and Cellular Biology 19: 5237–5246.
Catena, R., E. Escoffier, C. Caron, S. Khochbin, I. Martianov, and I. Davidson. 2009. HMGB4, a novel member of the HMGB family, is preferentially expressed in the mouse testis and localizes to the basal pole of elongating spermatids. Biology of Reproduction 80: 358–366.
Funding
This work was funded by the Jiangsu Youth Medical Talents Project (QNRC2016255) and Science Foundation of Bayinguolengmengguzizhizhou People’s Hospital (BHS201913).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zuo, T., Yue, Y., Wang, X. et al. Luteolin Relieved DSS-Induced Colitis in Mice via HMGB1-TLR-NF-κB Signaling Pathway. Inflammation 44, 570–579 (2021). https://doi.org/10.1007/s10753-020-01354-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-020-01354-2