Abstract—
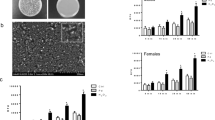
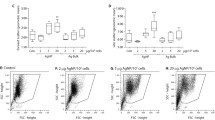
Apoptosis is an important cell death mechanism for the resolution of inflammation. Neutrophil spontaneous apoptosis rates were reported to be slightly different in men and women and to be modulated by female sex hormones. The aim of this study was to determine whether different nanoparticles (NPs) will alter the neutrophil and eosinophil apoptotic rates differently in men and women. Using the antiapoptotic cytokine granulocyte–macrophage colony-stimulating factor (GM-CSF) and the proapoptotic plant lectin Viscum album agglutinin-I (VAA-I) as controls, we found that these factors respectively delay and induce apoptosis in both neutrophils and eosinophils with apoptotic rates remarkably similar in both sexes. The polyamidoamine (PAMAM) dendrimers of generation 0 (G0) and G3 slightly, but not significantly, accelerate neutrophil apoptosis regardless of sex. Zinc oxide (ZnO), titanium dioxide (TiO2), cerium dioxide (CeO2), and palladium (Pd) but not platinum (Pt) NPs were found to significantly delay neutrophil apoptosis. When results were compared between men and women, only ZnO and Pd NPs were found to significantly delay neutrophil apoptosis in men while ZnO, TiO2, CeO2, and Pt NPs inhibit apoptosis in women neutrophils. In eosinophils, G3, but not G0 NPs, significantly accelerate apoptosis in women. ZnO, Pt, and Pd NPs significantly delay eosinophil apoptosis but only in women. Unlike neutrophils, TiO2 and CeO2 NPs did not significantly delay eosinophil apoptosis. We propose that future studies aiming at determining potential effect NPs on cellular biological processes should incorporate a sex-based analysis based on the differences reported here studying the impact of NPs on human granulocyte apoptosis.






Similar content being viewed by others
Availability of Data and Materials
Not applicable.
Code Availability
Not applicable.
References
Jaillon, S., K. Berthenet, and C. Garlanda. 2019. Sexual dimorphism in innate immunity. Clinical reviews in allergy & immunology 56: 308–321.
Lefevre, N., F. Corazza, J. Valsamis, A. Delbaere, V. De Maertelaer, J. Duchateau, and G. Casimir. 2019. The number of X chromosomes influences inflammatory cytokine production following toll-like receptor stimulation. Frontiers in immunology 10: 1052.
Liew, P.X., and P. Kubes. 2019. The neutrophil’s role during health and disease. Physiological reviews 99: 1223–1248.
Ratajczak-Wrona, W., K. Nowak, M. Garley, K. Grubczak, D. Dabrowska, A. Iwaniuk, S. Wilk, M. Moniuszko, J. Czerniecki, S. Wolczynski, and E. Jablonska. 2019. Expression of serine proteases in neutrophils from women and men: regulation by endocrine disruptor bisphenol A. Environmental toxicology and pharmacology 71: 103212.
Ratajczak-Wrona, W., M. Garley, M. Rusak, K. Nowak, J. Czerniecki, K. Wolosewicz, M. Dabrowska, S. Wolczynski, P. Radziwon, and E. Jablonska. 2021. Sex-dependent dysregulation of human neutrophil responses by bisphenol A. Environmental health 20: 5.
Pace, S., A. Rossi, V. Krauth, F. Dehm, F. Troisi, R. Bilancia, C. Weinigel, S. Rummler, O. Werz, and L. Sautebin. 2017. Sex differences in prostaglandin biosynthesis in neutrophils during acute inflammation. Scientific reports 7: 3759.
Brach, M.A., S. deVos, H.J. Gruss, and F. Herrmann. 1992. Prolongation of survival of human polymorphonuclear neutrophils by granulocyte-macrophage colony-stimulating factor is caused by inhibition of programmed cell death. Blood 80: 2920–2924.
Lavastre, V., M. Pelletier, R. Saller, K. Hostanska, and D. Girard. 2002. Mechanisms involved in spontaneous and Viscum album agglutinin-I-induced human neutrophil apoptosis: Viscum album agglutinin-I accelerates the loss of antiapoptotic Mcl-1 expression and the degradation of cytoskeletal paxillin and vimentin proteins via caspases. Journal of immunology 168: 1419–1427.
Babin, K., F. Antoine, D.M. Goncalves, and D. Girard. 2013. TiO2, CeO2 and ZnO nanoparticles and modulation of the degranulation process in human neutrophils. Toxicology letters 221: 57–63.
Chhay, P., M. Murphy-Marion, Y. Samson, and D. Girard. 2018. Activation of human eosinophils with palladium nanoparticles (Pd NPs): Importance of the actin cytoskeleton in Pd NPs-induced cellular adhesion. Environmental toxicology and pharmacology 57: 95–103.
Durocher, I., and D. Girard. 2016. In vivo proinflammatory activity of generations 0–3 (G0–G3) polyamidoamine (PAMAM) nanoparticles. Inflammation research 65: 745–755.
Goncalves, D.M., S. Chiasson, and D. Girard. 2010. Activation of human neutrophils by titanium dioxide (TiO2) nanoparticles. Toxicology in vitro 24: 1002–1008.
Goncalves, D.M., and D. Girard. 2014. Zinc oxide nanoparticles delay human neutrophil apoptosis by a de novo protein synthesis-dependent and reactive oxygen species-independent mechanism. Toxicology in vitro 28: 926–931.
Murphy-Marion, M., and D. Girard. 2018. Titanium dioxide nanoparticles induce human eosinophil adhesion onto endothelial EA.hy926 cells via activation of phosphoinositide 3-kinase/Akt cell signalling pathway. Immunobiology 223: 162–170.
Silva, L.R., and D. Girard. 2016. Human eosinophils are direct targets to nanoparticles: Zinc oxide nanoparticles (ZnO) delay apoptosis and increase the production of the pro-inflammatory cytokines IL-1beta and IL-8. Toxicology letters 259: 11–20.
Lavastre, V., S. Chiasson, H. Cavalli, and D. Girard. 2005. Viscum album agglutinin-I induces apoptosis and degradation of cytoskeletal proteins via caspases in human leukaemia eosinophil AML14.3D10 cells: Differences with purified human eosinophils. British Journal of Haematology 130: 527–535.
Noel, C., J.C. Simard, and D. Girard. 2016. Gold nanoparticles induce apoptosis, endoplasmic reticulum stress events and cleavage of cytoskeletal proteins in human neutrophils. Toxicology in vitro 31: 12–22.
Simard, J.C., M.M. Simon, P.A. Tessier, and D. Girard. 2011. Damage-associated molecular pattern S100A9 increases bactericidal activity of human neutrophils by enhancing phagocytosis. Journal of immunology 186: 3622–3631.
Bohmer, N., A. Rippl, S. May, A. Walter, M.B. Heo, M. Kwak, M. Roesslein, N.W. Song, P. Wick, and C. Hirsch. 2018. Interference of engineered nanomaterials in flow cytometry: A case study. Colloids and surfaces. B, Biointerfaces 172: 635–645.
Molloy, E.J., A.J. O’Neill, J.J. Grantham, M. Sheridan-Pereira, J.M. Fitzpatrick, D.W. Webb, and R.W. Watson. 2003. Sex-specific alterations in neutrophil apoptosis: The role of estradiol and progesterone. Blood 102: 2653–2659.
Fresneda Alarcon, M., Z. McLaren, and H. L. Wright. 2021. Neutrophils in the pathogenesis of rheumatoid arthritis and systemic lupus erythematosus: same foe different M.O. Frontiers in immunology 12: 649693.
Pérez-Figueroa, E., P. Álvarez-Carrasco, E. Ortega, and C. Maldonado-Bernal. 2021. Neutrophils: many ways to die. Frontiers in immunology 12: 631821.
Shi, J., and J. Li. 2020. Neutrophil-targeted engineered prodrug nanoparticles for anti-inflammation. FASEB journal 34: 9828–9831.
Xia, Q., J. Huang, Q. Feng, X. Chen, X. Liu, X. Li, T. Zhang, S. Xiao, H. Li, Z. Zhong, and K. Xiao. 2019. Size- and cell type-dependent cellular uptake, cytotoxicity and in vivo distribution of gold nanoparticles. International journal of nanomedicine 14: 6957–6970.
Zhang, C.Y., X. Dong, J. Gao, W. Lin, Z. Liu, and Z. Wang. 2019. Nanoparticle-induced neutrophil apoptosis increases survival in sepsis and alleviates neurological damage in stroke. Science advances 5: eaax7964.
Durocher, I., C. Noel, V. Lavastre, and D. Girard. 2017. Evaluation of the in vitro and in vivo proinflammatory activities of gold (+) and gold (-) nanoparticles. Inflammation research 66: 981–992.
Kwemo, P.S.A., M. Vanharen, I. Durocher, D. Girard. 2020. Impact of palladium nanoparticles (Pd-NPs) on the biology of neutrophils in vitro and on leukocyte attraction in vivo. Journal of Nanoparticle Research 22.
Liz, R., J.C. Simard, L.B. Leonardi, and D. Girard. 2015. Silver nanoparticles rapidly induce atypical human neutrophil cell death by a process involving inflammatory caspases and reactive oxygen species and induce neutrophil extracellular traps release upon cell adhesion. International immunopharmacology 28: 616–625.
Poirier, M., J.C. Simard, F. Antoine, and D. Girard. 2014. Interaction between silver nanoparticles of 20 nm (AgNP20) and human neutrophils: Induction of apoptosis and inhibition of de novo protein synthesis by AgNP20 aggregates. Journal of applied toxicology 34: 404–412.
Poirier, M., J. C. Simard, and D. Girard. 2015. Silver nanoparticles of 70 nm and 20 nm affect differently the biology of human neutrophils. J Immunotoxicol 1–11.
Thomas, C.J., and K. Schroder. 2013. Pattern recognition receptor function in neutrophils. Trends in immunology 34: 317–328.
Kvarnhammar, A.M., and L.O. Cardell. 2012. Pattern-recognition receptors in human eosinophils. Immunology 136: 11–20.
Pallardy, M.J., I. Turbica, and A. Biola-Vidamment. 2017. Why the immune system should be concerned by nanomaterials? Frontiers in immunology 8: 544.
Behzadi, S., V. Serpooshan, W. Tao, M.A. Hamaly, M.Y. Alkawareek, E.C. Dreaden, D. Brown, A.M. Alkilany, O.C. Farokhzad, and M. Mahmoudi. 2017. Cellular uptake of nanoparticles: Journey inside the cell. Chemical Society reviews 46: 4218–4244.
Arruda, M.A., and C. Barja-Fidalgo. 2009. NADPH oxidase activity: In the crossroad of neutrophil life and death. Frontiers in bioscience 14: 4546–4556.
Pujari-Palmer, S., S. Chen, S. Rubino, H. Weng, W. Xia, H. Engqvist, L. Tang, and M.K. Ott. 2016. In vivo and in vitro evaluation of hydroxyapatite nanoparticle morphology on the acute inflammatory response. Biomaterials 90: 1–11.
Couto, D., M. Freitas, V. Vilas-Boas, I. Dias, G. Porto, M.A. Lopez-Quintela, J. Rivas, P. Freitas, F. Carvalho, and E. Fernandes. 2014. Interaction of polyacrylic acid coated and non-coated iron oxide nanoparticles with human neutrophils. Toxicology letters 225: 57–65.
Simon, H.U. 2001. Regulation of eosinophil and neutrophil apoptosis–similarities and differences. Immunological reviews 179: 156–162.
Daigle, I., and H.U. Simon. 2001. Critical role for caspases 3 and 8 in neutrophil but not eosinophil apoptosis. International archives of allergy and immunology 126: 147–156.
Liles, W.C., D.C. Dale, and S.J. Klebanoff. 1995. Glucocorticoids inhibit apoptosis of human neutrophils. Blood 86: 3181–3188.
McColl, A., S. Michlewska, I. Dransfield, and A.G. Rossi. 2007. Effects of glucocorticoids on apoptosis and clearance of apoptotic cells. TheScientificWorldJOURNAL 7: 1165–1181.
Meagher, L.C., J.M. Cousin, J.R. Seckl, and C. Haslett. 1996. Opposing effects of glucocorticoids on the rate of apoptosis in neutrophilic and eosinophilic granulocytes. Journal of immunology 156: 4422–4428.
Taylor, E.L., I.L. Megson, C. Haslett, and A.G. Rossi. 2003. Nitric oxide: A key regulator of myeloid inflammatory cell apoptosis. Cell death and differentiation 10: 418–430.
Vanharen, M., and D. Girard. 2020. Activation of human eosinophils with nanoparticles: A new area of research. Inflammation 43: 8–16.
Janaszewska, A., M. Ciolkowski, D. Wróbel, J.F. Petersen, M. Ficker, J.B. Christensen, M. Bryszewska, and B. Klajnert. 2013. Modified PAMAM dendrimer with 4-carbomethoxypyrrolidone surface groups reveals negligible toxicity against three rodent cell-lines. Nanomedicine : Nanotechnology, biology, and medicine 9: 461–464.
Mukherjee, S.P., F.M. Lyng, A. Garcia, M. Davoren, and H.J. Byrne. 2010. Mechanistic studies of in vitro cytotoxicity of poly(amidoamine) dendrimers in mammalian cells. Toxicology and applied pharmacology 248: 259–268.
Naha, P.C., M. Davoren, F.M. Lyng, and H.J. Byrne. 2010. Reactive oxygen species (ROS) induced cytokine production and cytotoxicity of PAMAM dendrimers in J774A.1 cells. Toxicology and applied pharmacology 246: 91–99.
Miyagi, M., H. Aoyama, M. Morishita, and Y. Iwamoto. 1992. Effects of sex hormones on chemotaxis of human peripheral polymorphonuclear leukocytes and monocytes. Journal of periodontology 63: 28–32.
Bouman, A., M.J. Heineman, and M.M. Faas. 2005. Sex hormones and the immune response in humans. Human reproduction update 11: 411–423.
Ray, J. L., P. Fletcher, R. Burmeister, and A. Holian. 2020. The role of sex in particle-induced inflammation and injury. Wiley interdisciplinary reviews. Nanomedicine and nanobiotechnology 12: e1589.
Funding
The study was supported by grants (2017–0044) from the Institut de recherche Robert-Sauvé en santé et en sécurité du travail (IRSST).
Author information
Authors and Affiliations
Contributions
MV, ID, and AS carried out the experiments and plotted and analyzed the data. DG wrote the manuscript with support from MV, ID, and AS and is the supervisor of the project.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vanharen, M., Durocher, I., Saafane, A. et al. Evaluating the Apoptotic Cell Death Modulatory Activity of Nanoparticles in Men and Women Neutrophils and Eosinophils. Inflammation 45, 387–398 (2022). https://doi.org/10.1007/s10753-021-01553-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-021-01553-5