Abstract
In this article, the thermophysical properties of binary eutectic PCM salt (LiNO3 + NaCl) are investigated experimentally using the dispersion of multi-walled carbon nanotubes (MWCNTs) with varying weight fractions (i.e., 0.25 %, 0.5 %, and 1 %). According to the XRD and FTIR results, MWCNTs physically amalgamated with base eutectic salt without affecting their chemical structure. The Kissinger model is used to assess the phase change kinetics of the prepared nano-PCM composites. With the addition of MWCNTs, the activation energy of the chosen PCM is significantly increased. The thermophysical properties of the nano-PCM samples, such as phase transition temperature and latent heat value, are measured using differential scanning calorimetry (DSC) and thermal conductivity using a laser flash apparatus. The results show that dispersing 1 % MWCNTs in PCM salt improved the thermal conductivity enhancement ratio by 38.59 %, while decreasing the latent heat storage capacity by 14.98 %. Furthermore, by training the experimental DSC values of nano-PCM samples at various heating rates, an artificial neural network is developed to predict the thermophysical properties of nanocomposites. With an R2 value of 0.998, the developed neural network accurately predicted the experimental DSC values.





















Similar content being viewed by others
References
M. Senthilkumar, K.R. Balasubramanian, R.K. Kottala, S.P. Sivapirakasam, L. Maheswari, J. Therm. Anal. Calorim. 141, 2487 (2020)
S.K. Marudaipillai, B. Karuppudayar Ramaraj, R.K. Kottala, M. Lakshmanan, Energy Sources Part A Recover Util. Environ. Eff. (2020). https://doi.org/10.1080/15567036.2020.1806409
B.S. Jinshah, B.K. Ramaraj, R.K. Kottala, S.P. Sivapirakasam, Energy Sources Part A Recover. Util. Environ. Eff. (2021). https://doi.org/10.1080/15567036.2021.1944403
K. Pielichowska, K. Pielichowski, Prog. Mater. Sci. 65, 67 (2014)
N. Beemkumar, A. Karthikeyan, D. Yuvarajan, S. Lakshmi-Sankar, Arab. J. Sci. Eng. 42, 2055 (2017)
D. Kim, J. Jung, Y. Kim, M. Lee, J. Seo, S.B. Khan, Int. J. Heat Mass Transf. 95, 735 (2016)
Z. Li, W.G. Sun, G. Wang, Z.G. Wu, Sol. Energy Mater. Sol. Cells 128, 447 (2014)
R. Wen, Z. Huang, Y. Huang, X. Zhang, X. Min, M. Fang, Y. Liu, X. Wu, Energy Build. 116, 677 (2016)
Z. Liu, Y. Zhang, K. Hu, Y. Xiao, J. Wang, C. Zhou, J. Lei, Energy Build. 127, 327 (2016)
Y. Deng, J. Li, T. Qian, W. Guan, X. Wang, J. Mater. Sci. Technol. 33, 198 (2017)
Y.B. Tao, C.H. Lin, Y.L. He, Energy Convers. Manag. 97, 103 (2015)
H. Tian, W. Wang, J. Ding, X. Wei, C. Huang, Sol. Energy Mater. Sol. Cells 149, 187 (2016)
Y. Qin, G. Leng, X. Yu, H. Cao, G. Qiao, Y. Dai, Y. Zhang, Y. Ding, Powder Technol. 282, 37 (2015)
L. Zhong, X. Zhang, Y. Luan, G. Wang, Y. Feng, D. Feng, Sol. Energy 107, 63 (2014)
M.M. Kenisarin, Renew. Sustain. Energy Rev. 14, 955 (2010)
C. Zhu, X. Cheng, Y. Li, B. Tao, Sol. Energy 118, 303 (2015)
R.I. Olivares, Sol. Energy 86, 2576 (2012)
Y. Ren, C. Xu, M. Yuan, F. Ye, X. Ju, X. Du, Energy Convers. Manag. 163, 50 (2018)
D. Zhou, P. Eames, Sol. Energy Mater. Sol. Cells 167, 157 (2017)
L. Han, S. Xie, S. Liu, J. Sun, Y. Jia, Y. Jing, Appl. Energy 185, 762 (2017)
G. Feng, X. Xu, N. He, H. Li, K. Huang, Mater. Res. Innov. 19, S5972 (2015)
S.C. Lin, H.H. Al-Kayiem, Sol. Energy 132, 267 (2016)
A.J. Chamkha, A. Doostanidezfuli, E. Izadpanahi, M. Ghalambaz, Adv. Powder Technol. 28, 385 (2017)
F. Ye, Z. Ge, Y. Ding, J. Yang, Particuology 15, 56 (2014)
L.S. Wong-Pinto, Y. Milian, S. Ushak, Renew. Sustain. Energy Rev. 122, 109727 (2020)
S.C. Kim, R. Prabakaran, D. Sakthivadivel, N. Thangapandian, A. Bhatia, P. Ganesh Kumar, Fullerenes Nanotub. Carbon Nanostruct. 28, 925 (2020)
V. Kumaresan, K.S. Raghavan, M.P. Vikram, J. Iyyappan, Fullerenes Nanotub. Carbon Nanostruct. 29, 670 (2021)
S. Salyan, S. Suresh, Adv. Powder Technol. 29, 3183 (2018)
N. Putra, S. Rawi, M. Amin, E. Kusrini, E.A. Kosasih, T.M. Indra Mahlia, J. Energy Stor. 21, 32 (2019)
Y. Harmen, Y. Chhiti, F.E. M’Hamdi Alaoui, F. Bentiss, C. Jama, S. Duquesne, M. Bensitel, Fullerenes Nanotub. Carbon Nanostruct. 29, 732 (2021)
T. Wu, N. Xie, J. Niu, J. Luo, X. Gao, Y. Fang, Z. Zhang, Int. J. Refrig. 113, 136 (2020)
W. Zhou, J. Jiang, H. Wu, D. Hu, P. Li, X. Yang, X. Jia, ACS Appl. Energy Mater. 4, 4561 (2021)
D. Toghraie, M.H. Aghahadi, N. Sina, F. Soltani, Int. J. Thermophys. 41, 1 (2020)
A. Karimipour, O. Malekahmadi, A. Karimipour, M. Shahgholi, Z. Li, Int. J. Thermophys. 41, 1 (2020)
S. Sunphorka, B. Chalermsinsuwan, P. Piumsomboon, Fuel 193, 142 (2017)
A. Hai, G. Bharath, M. Daud, K. Rambabu, I. Ali, S.W. Hasan, P.L. Show, F. Banat, Chemosphere 283, 131162 (2021)
S.U. Ilyas, R. Pendyala, M. Narahari, L. Susin, Energy Convers. Manag. 142, 215 (2017)
H.E. Kissinger, Anal. Chem. 29, 1702 (1957)
S. Vyazovkin, Molecules 25, 2813 (2020)
L. Feng, J. Zheng, H. Yang, Y. Guo, W. Li, X. Li, Sol. Energy Mater. Sol. Cells 95, 644 (2011)
A. Palacios, L. Cong, M.E. Navarro, Y. Ding, C. Barreneche, Renew. Sustain. Energy Rev. 108, 32 (2019)
E. Hamdy, S. Ebrahim, F. Abulfotuh, M. Soliman, in Proceedings of 2016 International Renewable and Sustainable Energy Conference (IRSEC 2016), p. 317 (2017)
M. Vivekananthan, V.A. Amirtham, Thermochim. Acta 676, 94 (2019)
R.P. Singh, J.Y. Sze, S.C. Kaushik, D. Rakshit, A. Romagnoli, J. Energy Stor. 33, 102092 (2021)
Z. Zhang, Y. Yuan, S. Alelyani, X. Cao, P.E. Phelan, Sol. Energy 155, 661 (2017)
Acknowledgements
This work is carried out at the National Institute of Technology in Tiruchirappalli, Tamilnadu, India, and thankful to the Institute for providing us with access to the all-research facilities. The authors would also like to express their gratitude to the Ministry of Education, Government of India, for providing a stipend.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix 1
Appendix 1
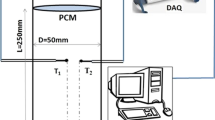
1.1 Laser Flash Apparatus (LFA)
Thermal conductivity is a key thermophysical parameters for characterizing LiNO3 + NaCl binary eutectic PCM with different MWCNT compositions. The thermal conductivity was measured using a NETZSCH 467 laser flash instrument with an accuracy of ± 2 % within the range of 0.1–2000 W·mK−1. The laser flash apparatus operated in the presence of a thermally insulated infinite slab that was uniformly subjected to a radiant energy heat pulse over the front surface. The instantaneous energy pulse was induced on the front surface of the sample by irradiating the laser beam in the laser flash method. The laser energy absorbed by the sample's front surface was transformed to heat energy, which spread throughout the sample. A photovoltaic infrared detector was used to measure the temperature of the sample's back surface. The temperature of the sample's back surface is used to compute the thermal diffusivity. The Fourier series can be used to express the temperature history of the back surface.
QR = Radiant energy per unit surface area (J·m−2), ρPCM = density of the PCM sample (kg·m−3), \({C}_{{P}_{PCM}}\) = Specific heat capacity of the PCM (J·kg−1 K−1), τ = thickness of the sample (m).
The thermal diffusivity of each sample can be computed by following equation:
The following equation can be used to compute the mass density value of nano-dispersed PCM samples. Meanwhile, the specific heat capacity of the sample can be obtained through DSC data.
Finally, thermal conductivity of sample can be determined by following expression:
The uncertainty of the thermal conductivity using LFA method was estimated based on the following parameters, i.e., thermal diffusivity (\(\alpha\)), density (\(\rho\)), and specific heat capacity (\({C}_{P}\)).
The uncertainty of the thermal conductivity can be determined by following equation:
Using the above expression, the uncertainty of the thermal conductivity is calculated as 4.2 %.
1.2 Sample Preparation
Initially, an equal amount (i.e., 2gm) of prepared samples was inserted in the hydraulic press and uniform pressure was applied all around until the cylindrical-shaped pallets were formed. To determine thermal conductivity, materials were compressed into pellets with a diameter of 25 mm and a thickness of 3 mm using a hydraulic press at 8Mpa. The prepared samples are shown in Fig. 22.
1.3 KD2 Pro Thermal Conductivity Analyzer
The KD2 Pro thermal conductivity analyzer is used to measure thermal conductivity of the prepared nano-PCM samples. TR-1 needle sensor is used to measure the thermal conductivity of PCM and nano-dispersed PCMs. The following points should be noted before conducting the experiment:
-
Insert the TR-1 needle into the standard verification block to calibrate the equipment, and repeat the experiment at least four times to estimate the equipment’s accuracy.
-
Prepare the PCM samples according to ASTM 5334 guidelines, before inserting the needle.
-
Hold the needle for at least 15 min to get more accurate results.
-
Thermal grease is applied to the needle to reduce the thermal contact resistance between the sample and the needle.
Before measuring the thermal conductivity of the PCM sample, it is necessary to calibrate the instrument using standard verification gauge. Measure the thermal conductivity of the verification standard gauge and validate with the certified value (Fig. 23). After calibration, the samples are allowed to measure the thermal conductivity using TR-1 sensor (Fig. 24).
1.4 Measurement of Thermal Conductivity Using TR-1 Sensor
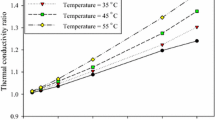
The thermal conductivity of PCM was measured using the TR-1 needle after it was filled in a centrifuge tube. PCM is found to have a thermal conductivity of 1.551 W·mK−1 (Fig. 25). The thermal conductivity of 1 % MWCNT PCM was measured using a TR-1 needle after it was poured and compressed in the centrifuge tube. The thermal conductivity data are shown in Fig. 25, and it is found that the thermal conductivity of 1 % MWCNT PCM sample is 2.1723 W·mK−1, which is 40 % greater than that of pure PCM. The following result confirms that adding MWCNT increased thermal conductivity greatly, which would improve the PCM’s thermal response even more.
The measured thermal conductivities of prepared PCM samples using LFA and KD2 Pro thermal conductivity analyzers are given in Table
7. The error deviation between these two methods for measure the PCM samples like pure PCM, 0.25 % MWCNT, 0.5 % MWCNT, and 1 % MWCNT are 5 %, 4.5 %, 3 %, and 4 %, respectively.
Similarly, the sample after thermal durability test was measured using LFA and KD2 Pro thermal conductivity approaches and results are listed in Table
8. The error deviation between these two methods for measure the PCM samples like pure PCM and 1 % MWCNT are 4.7 % and 4 %, respectively. From the results, it is observed that LFA method is most effectively used to measure the thermal conductivity of the PCM samples as compared with the KD2 Pro method.
Rights and permissions
About this article
Cite this article
Ravi Kumar, K., Balasubramanian, K.R., Jinshah, B.S. et al. Experimental Analysis and Neural Network Model of MWCNTs Enhanced Phase Change Materials. Int J Thermophys 43, 11 (2022). https://doi.org/10.1007/s10765-021-02937-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10765-021-02937-3