Abstract
Introduction
Presently the techniques for making transgenic animals are cumbersome, required costly instruments and trained man-power. The ability of spermatogonial stem cells (SSCs) to integrate foreign genes has provided the opportunity for developing alternate methods for generation of transgenic animals. One of the big challenges in this field is development of the methods to identify and purify donor SSCs by antibody mediated cell sorting.
Purpose
The present study was aimed to identify goat subpopulations of SSCs using polyclonal antibodies against PGP9.5 and c-kit molecular markers as well as the growth characteristics of SSCs during short term culture.
Methods
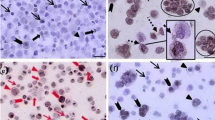
One month old goats’ testicular samples were subjected for immunohistochemical and immunocytochemical evaluations. The enzymatically isolated SSCs were cultured in DMEM plus FCS supplemented with (treatment) or without (control) growth factors (GDNF, LIF, FGF, and EGF) for 2 weeks. At the end of culture the morphological characteristics of SSCs colonies and immunocytochemical staining were evaluated.
Results
The number and size of colonies in treatment groups were significantly (P < 0.01) higher than corresponding values in controls. The presence of PGP 9.5 and c-kit antigens was confirmed in immunocytochemical evaluation. In immunocytochemical evaluation, the proportion of c-kit and PGP9.5 positive cells were significantly (P < 0.001) higher in control and treatment groups, respectively.
Conclusions
The presence of PGP9.5 and c-kit antigens was confirmed in goat SSCs. Moreover, culture medium supplementation with growth factors could effectively retain the undifferentiation status of SSCs, reflected as a higher population of PGP9.5 positive cells, after short term culture.







Similar content being viewed by others
References
Van Pelt AMM, Rita Morena A, Van Dissel-Emiliani FMF, Boitani C, Gaemers IC, De Rooij DG, et al. Isolation of the synchronized A spermatogonia from adult vitamin A-deficient rat testes. Biol Reprod. 1996;55:439–44.
Russell L, Ettlin R, Sinha-Hikim A, Clegg E, Russell L, Ettlin R, Sinha Hikim A, Clegg E. Histological and histopathological evaluation of the testis. 1st ed. Clearwater: Cache River; 1990.
Dobrinski I. Transplantation of germ cells and testis tissue to study mammalian spermatogenesis. Anim Reprod. 2006;3:135–45.
Tegelenbosch RA, de Rooij DG. A quantitative study of spermatogonial multiplication and stem cell renewal in the C3H/101 F1 hybrid mouse. Mutat Res. 1993;290:193–200.
Huckins C. The spermatogonial stem cell population in adult rats. Their morphology, proliferation and maturation. Anat Rec. 1971;169:533–58.
Clermont Y. Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonial renewal. Physiol Rev. 1972;52:198–236.
Meistrich ML, van Beek M. Spermatogonial stem cells. In: Desjardins C, Ewing LL, editors. Cell and molecular biology of the testis. New York: Oxford University Press; 1993. p. 266–95.
Aponte PM, van Bragt MP, de Rooij DG, van Pelt AM. Spermatogonial stem cells: characteristics and experimental possibilities. APMIS. 2005;113:727–42.
Izadyar F, Ouden K, Creemers LB, Posthuma G, Parvinen M, de Rooij DG. Proliferation and differentiation of bovine type A spermatogonia during long-term culture. Biol Reprod. 2003;68:272–81.
Phillips BT, Gassei K, Orwig KE. Spermatogonial stem cell regulation and spermatogenesis. Phil Trans R Soc B. 2010;365:1663–78.
Kanatsu-Shinohara M, Ogonuki N, Inoue K, Miki H, Ogura A, Toyokuni S, Shinohara T. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol Reprod. 2003;69:612–6.
Schrans-stasseen BHGJ, van de Kant HJG, de Rooij DG, van Pelt AMM. Differential expression of c-kit in mouse undifferentiated and differentiating type A spermatogonia. Endocrinology. 1999;140:5894–900.
Izadyar F, Spierenberg GT, Creemers LB, den Ouden K, de Rooij DG. Isolation and purification of type A spermatogonia from the bovine testis. Reproduction. 2002;124:85–94.
Rodriguez-Sosa JR, Dobson H, Hahnel A. Isolation and transplantation of spermatogonia in sheep. Theriogenology. 2006;66:2091–103.
Yasuhiro K, Daiji E, Toshihiko I. Expression of protein gene product 9.5, a neuronal ubiquitin C-terminal hydrolase, and its developing change in sertoli cells of mouse testis. Mol Reprod Dev. 1999;54:333–41.
Zhang Z, Hill J, Holland M, Kurihara Y, Loveland KL. Bovine sertoli cells colonize and form tubules in murine hosts following transplantation and grafting procedures. J Androl. 2008;29:418–30.
Von Kopylow K, Kirchhoff C, Jezek D, Schulze W, Feig C, Primig M, et al. Screening for biomarkers of spermatogonia within the human testis: a whole genome approach. Hum Reprod. 2010;25:1104–12.
Zeng W, Snedaker AK, Megee S, Rathi R, Chen F, Honaramooz A, et al. Preservation and transplantation of porcine testis tissue. Reprod Fertile Dev. 2009;21:489–97.
Hofmann MC, Braydich-Stollea L, Dym M Isolation of male germ-line stem cells; influence of GDNF. Dev Biol. 2005;279:114–24.
Roosen-Runge EC, Giesel JLO. Quantitative studies on spermatogenesis in the albino rat. Am J Anat. 1950;87:1–30.
Monesi V. Autoradiographic study of DNA synthesis and the cell cycle in spermatogonia and spermatocytes of mouse testis using tritiated thymidine. J Cell Biol. 1962;14:1–18.
Lok D, Weenk D, de Rooij DG. Morphology, proliferation and differentiation of undifferentiated spermatogonia in the Chinese hamster and the ram. Anat Rec. 1982;203:83–99.
Shinohara T, Orwig KE, Avarbock MR, Brinster RL. Spermatogonial stem cell enrichment by multiparameter selection of mouse testis. Proc Natl Acad Sci. 2000;97:8346–51.
Wrobel KH, Bickel D, Kujat R, Schimmel M. Configuration and distribution of bovine spermatogonia. Cell and Tissue Res. 1995;279:277–89.
Herrid M, Olejnik J, Jackson M, Suchowerska N, Stockwell S, Davey R, et al. Irradiation enhances the efficiency of testicular germ cell transplantation in sheep. Biol Reprod. 2009;81:898–905.
Luo J, Megee S, Rathi R, Dobrinski I. Protein gene product 9.5 is a spermatogonia-specific marker in the pig testis: application to enrichment and culture of porcine spermatogonia. Mol Reprod Dev. 2006;73:1531–40.
Young HS, Kumar GM, Lee USJ, Taek H. Isolation and in vitro culture of pig spermatogonial stem cell. Asian-Aust J Anim Sci. 2009;22:187–93.
Acknowledgment
The authors would like to thank the Avicenna Research Institute for technical and financial supports, ACECR, Tehran, Dr. K. Kamali for assistance with the statistical analysis, and Dr. H. Soltangharaee for histological evaluation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule
This study confirmed the different expression of PGP9.5 and c-kit markers in subpopulation of undifferentiated and differentiated goat type A spermatogonia.
Rights and permissions
About this article
Cite this article
Heidari, B., Rahmati-Ahmadabadi, M., Akhondi, M.M. et al. Isolation, identification, and culture of goat spermatogonial stem cells using c-kit and PGP9.5 markers. J Assist Reprod Genet 29, 1029–1038 (2012). https://doi.org/10.1007/s10815-012-9828-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-012-9828-5