Abstract
In the previous paper, we reviewed the origins of energy based calculations, and the early science of FF development. The initial efforts spanning the period from roughly the early 1970s to the mid to late 1990s saw the development of methodologies and philosophies of the derivation of FFs. The use of Cartesian coordinates, derivation of the H-bond potential, different functional forms including diagonal quadratic expressions, coupled valence FFs, functional form of combination rules, and out of plane angles, were all investigated in this period. The use of conformational energetics, vibrational frequencies, crystal structure and energetics, liquid properties, and ab initio data were all described to one degree or another in deriving and validating both the FF functional forms and force constants. Here we discuss the advances made since in improving the rigor and robustness of these initial FFs. The inability of the simple quadratic diagonal FF to accurately describe biomolecular energetics over a large domain of molecular structure, and intermolecular configurations, was pointed out in numerous studies. Two main approaches have been taken to overcome this problem. The first involves the introduction of error functions, either exploiting torsion terms or introducing explicit 2-D error correction grids. The results and remaining challenges of these functional forms is examined. The second approach has been to improve the representation of the physics of intra and intermolecular interactions. The latter involves including descriptions of polarizability, charge flux aka geometry dependent charges, more accurate representations of spatial electron density such as multipole moments, anisotropic nonbond potentials, nonbond and polarization flux, among others. These effects, though not as extensively studied, likely hold the key to achieving the rigorous reproduction of structural and energetic properties long sought in biomolecular simulations, and are surveyed here. In addition, the quality of training and validation observables are evaluated. The necessity of including an ample set of energetic and crystal observables is emphasized, and the inadequacy of free energy as a criterion for FF reliability discussed. Finally, in light of the results of applications of the two approaches to FF development, we propose a “recipe” of terms describing the physics of inter and intramolecular interactions whose inclusion in FFs would significantly improve our understanding of the energetics and dynamics of biomolecular systems resulting from molecular dynamics and other energy based simulations.

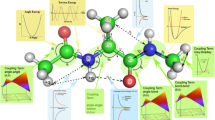
Reproduced with permission from Debiec et al. [58]

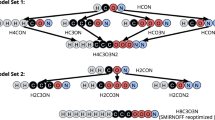
Reproduced with permission from Huang et al. supplementary material [65]

Reproduced with permission Graphical abstract. Schmollngruber et al. [149]


Similar content being viewed by others
Abbreviations
- AG:
-
Arithmetic–geometric
- Ala:
-
Alanine
- AMBER:
-
Assisted model building with energy refinement
- AMOEBA:
-
Atomic multipole optimized energetics for biomolecular applications
- AUE:
-
Absoluter unsigned error
- BCC:
-
Bond charge correction
- BNS:
-
Ben Naim–Stillinger
- C22:
-
CHARMM22
- CASP:
-
Critical assessment of protein structure prediction
- CFF:
-
Consistent force field
- CHARMM:
-
Chemistry at HARvard Macromolecular Mechanics
- CHELPG:
-
Charges from electrostatic potentials using a grid-based method
- CHEQ:
-
Charge equilibration method
- CMAP:
-
Grid based energy correction map
- CNDO:
-
Complete neglect of differential overlap
- COMPASS:
-
Condensed-phase optimized molecular potentials for atomistic simulation studies
- CVFF:
-
Consistent valence force field
- DFT:
-
Density functional theory
- DMA:
-
Distributed multipole analysis
- DZVP:
-
Valence double-zeta plus polarization
- ECEPP:
-
Empirical conformational energy program for peptides
- EHT:
-
Extended Hückel theory
- ESP:
-
Electrostatic potential
- EVB:
-
Empirical valence bond
- FEP:
-
Free energy perturbation
- FF:
-
Force field
- FQ:
-
Fluctuating charges
- FRET:
-
Forster resonance energy transfer
- GAFF:
-
General AMBER force field
- Gly:
-
Glycine
- GROMOS:
-
GROningen MOlecular Simulation
- hCatL:
-
human Cathepsin L
- HF-SCF:
-
Hartree–Fock self-consistent-field
- Hyp:
-
Hydroxyproline
- IDP:
-
Intrinsically disordered protein
- LCAO:
-
Linear combination of atomic orbitals
- LJ:
-
Lennard-Jones
- LP:
-
Oxygen lone pair
- MC:
-
Monte Carlo
- MCMS FF:
-
Momany, Carruthers, McGuire, and Scheraga force field
- MCY:
-
Matsuoka–Clementi–Yoshimine
- MD:
-
Molecular dynamics
- MDDR:
-
MDL drug data report
- MDL:
-
Molecular Design Limited
- MEP:
-
Molecular electrostatic potentials
- MM:
-
Molecular mechanics
- MMFF:
-
Merck molecular force field
- NMA:
-
N-methylacetamide
- OPLS:
-
Optimized potential for liquid simulations
- OPLS-AA:
-
OPLS all atom
- OPLS-AA:
-
OPLS-AA/L OPLS all atom FF (L for LMP2)
- PCILO:
-
Perturbative configuration interaction using localized orbitals
- PDB:
-
Protein data base
- PEFC:
-
Potential Energy Function Consortium (Biosym)
- PMF:
-
Potential of mean force
- POL3:
-
Polarizable water model (3)
- PPII :
-
Polyproline II conformation
- QCPE:
-
Quantum chemistry program exchange
- QDF:
-
Quantum derivative fitting
- QDP:
-
Charge dependent polarizability
- QM:
-
Quantum mechanics
- RESP:
-
Restrained electrostatic potential
- RMS:
-
Root mean square
- RMSD:
-
Root mean square deviation
- RMSE:
-
Root mean square error
- SAMPL:
-
Statistical assessment of the modeling of proteins and ligands (competition)
- SAXS:
-
Small angle X-ray scattering
- SCF-LCAO-MO:
-
Self-consistent field-linear combination of atomic–molecular orbital
- SDFF:
-
Spectroscopically determined force fields (for macromolecules)
- SIBFA:
-
Sum of interactions between fragments ab initio (computed)
- SPC:
-
Simple point charge (water model)
- ST2:
-
Four point water model replacing Ben-Naim Stillinger (BNS) model
- STO:
-
Slater-type atomic orbitals
- SWM4-NDP:
-
Simple water model with negative Drude polarization
- TIP3P:
-
Transferable intermolecular potential three point
- TTBM:
-
Tri-tert-butylmethane
- TZVP:
-
Triple-zeta plus valence polarization (basis set)
- UB:
-
Urey–Bradley
- UBFF:
-
Urey Bradley force field
- VDW:
-
Van der Waals
- VFF:
-
Valence force field
- WH:
-
Waldman–Hagler
References
Dauber P, Hagler AT (2018) On the evolution of force fields for molecular mechanics and dynamics studies of biomolecular systems and drug design: where have we been, where are we now, where do we need to go and how do we get there? J Comput Aided Mol Des. https://doi.org/10.1007/s10822-018-0111-4
Westheimer FH (1956) Steric effects in organic chemistry. Wiley, New Jersey
Allinger NL (1959) Conformational analysis. III application to some medium ring compounds. J Am Chem Soc 81:5727–5733
Hendrickson JB (1961) Molecular geometry I. Machine computation of the common rings. J Am Chem Soc 83:4537–4547
Wiberg KB (1965) A scheme for strain energy minimization. Application to the cycloalkanes. J Am Chem Soc 87:1070–1078
Bixon M, Lifson S (1967) Potential functions and conformations in cycloalkanes. Tetrahedron 23:769–784
Lifson S, Warshel A (1968) Consistent force field for calculations of conformations, vibrational spectra, and enthalpies of cycloalkane and n-alkane molecules. J Chem Phys 49:5116
Lifson S (1973) Recent developments in consistent force field calulations. In: G. Sadron (ed) Dynamic aspects of conformations change in biological macromolecules. Springer, Dordrecht pp 421–430
Allinger NL, Miller MA, Vancatledge FA, Hirsch JA (1967) Conformational analysis. LVII. The calculation of the conformational structures of hydrocarbons by the Westheimer–Hendrickson–Wiberg method. J Am Chem Soc 89:4345–4357
Allinger NL, Tribble MT, Miller MA, Wertz DH (1971) Conformational analysis. LXIX. An improved calculations of the structures and energies of hydrocarbons. J Am Chem Soc 93:1637–1648
Scott RA, Scheraga HA (1966) Conformational analysis of macromolecules. I. ethane, propane, n-butane, and n-pentane. Biopolymers 4:237–238
Scott RA, Scheraga HA (1966) Conformational analysis of macromolecules. III. Helical structures of polyglycine and poly-l-alanine. J Chem Phys 45:2091–2101
Allinger NL (1976) Calculation of molecular structure and energy by force-field methods. Adv Phys Org Chem 13:1–82
Weiner PK, Kollman PA, AMBER (1981) Assisted model building with energy refinement. A general program for modeling molecules and their interactions. J Comput Chem 2:287–303
Hagler ATT, Dauber P, Lifson S (1979) Consistent force field studies of intermolecular forces in hydrogen-bonded crystals. 3. The C=O⋯H–O hydrogen bond and the analysis of the energetics and packing of carboxylic acids. J Am Chem Soc 101:5131–5141
Hagler AT, Huler E, Lifson S (1974) Energy functions for peptides and proteins. I. derivation of a consistent force field including the hydrogen bond from amide crystals. JAm Chem Soc 96:5319–5327
Dauber P, Osguthorpe DJ, Hagler AT, Structure (1982) Energetics and dynamics of ligand binding to dihydrofolate-reductase. Biochem 10:312–318
Brooks BR et al (1983) CHARMM: A program for macromolecular energy, minimization, and dynamics calculations. J Comput Chem 4:187–217
van Gunsteren WF, Berendsen HJC (1987) GROningen MOlecular simulation (GROMOS) library manual. BIOMOS, Nijenborgh. pp 1–229
Jorgensen WL, Tirado-Rives J (1988) The OPLS potential functions for proteins. Energy minimizations for crystals of cyclic peptides and crambin. J Am Chem Soc 110:1657–1666
Allinger NL (1977) Conformational analysis. 130. MM2. A hydrocarbon force field utilizing V1 and V2 torsional terms. J Am Chem Soc 99:8127–8134
MacKerell AD et al (1998) All-atom empirical potential for molecular modeling and dynamics studies of proteins. J Phys Chem B 102:3586–3616
Warshel A, Lifson S (1969) An empirical function for second neighbor interactions and its effect on vibrational modes and other properties of cyclo- and n-alkanes. Chem Phys Lett 4:255–256
Hagler AT, Lifson S, Dauber P (1979) Consistent force field studies of intermolecular forces in hydrogen-bonded crystals. 2. a benchmark for the objective comparison of alternative force fields. J Am Chem Soc 101:5122–5130
Dauber P, Hagler AT, Crystal Packing (1980) Hydrogen bonding, and the effect of crystal forces on molecular conformation. Acct Chem Res 13:105–112
Allinger NL, Yuh YH, Jenn-Huei L (1989) Molecular mechanics. The MM3 force field for hydrocarbons. J Am Chem Soc 11:8551–8566
Allinger NL, Chen K, Lii J (1996) An improved force field (MM4) for saturated hydrocarbons. J Comp Chem 17:642–668
Maple JR, Dinur U, Hagler AT (1988) Derivation of force fields for molecular mechanics and dynamics from ab initio energy surfaces. Proc Nat Acad Sci USA 85:5350–5354
Waldman M, Hagler ATT (1993) New combining rules for rare-gas van der Waals parameters. J Comput Chem 14:1077–1084
Lii J, Allinger NL (1991) The MM3 force field for amides, polypeptides and proteins. J Comput Chem 12:186–199
Weiner SJ, Kollman PA, Nguyen DT, Case DA (1986) An all atom force field for simulations of proteins and nucleic acids. J Comput Chem 7:230–252
Jorgensen WL, Maxwell DS, Tirado-Rives J (1996) Development and testing of the OPLS All-atom force field on conformational energetics and properties of organic liquids. J Am Chem Soc 118:11225–11236
Horta BAC et al (2016) A GROMOS-compatible force field for small organic molecules in the condensed phase: The 2016H66 parameter set. J Chem Theory Comput. https://doi.org/10.1021/acs.jctc.6b00187
Wang J, Cieplak P, Kollman PA (2000) How well does a restrained electrostatic potential (RESP) model perform in calculating conformational energies of organic and biological molecules? J Comput Chem 21:1049–1074
Wang JM, Wolf RM, Caldwell JW, Kollman PA, Case DA (2004) Development and testing of a general amber force field. J Comp Chem 25:1157–1174
Kaminski GA, Friesner RA, Tirado-rives J, Jorgensen WL (2001) Evaluation and reparametrization of the OPLS-AA force field for proteins via comparison with accurate quantum chemical calculations on peptides. J Phys Chem B 105:6474–6487
Cornell WD et al (1995) A second generation force field for the simulation of proteins, nucleic acids, and organic molecules. J Am Chem Soc 117:5179–5197
MacKerell AD, Feig M, Brooks CL (2004) Extending the treatment of backbone energetics in protein force fields: limitations of gas-phase quantum mechanics in reproducing protein conformational distributions in molecular dynamics simulations. J Comput Chem 25:1400–1415
Buck M, Bouguet-Bonnet S, Pastor RW, MacKerell AD (2006) Importance of the CMAP correction to the CHARMM22 protein force field: dynamics of hen lysozyme. Biophys J 90:L36–L38
Mu Y, Kosov DS, Stock G (2003) Conformational dynamics of trialanine in water. 2. comparison of AMBER, CHARMM, GROMOS, and OPLS force fields to NMR and infrared experiments. J Phys Chem B 107:5064–5073
Cardamone S, Hughes TJ, Popelier PL (2014) A. multipolar electrostatics. PhysChemChemPhys 16:10367–10387
Pierro M Di, Elber R (2013) Automated optimization of potential parameters. J Chem Theory Comput. https://doi.org/10.1021/ct400313n
Stone AJ (2013) The theory of intermolecular forces. Oxford University Press, Oxford
Hirschfelder JO, Curtiss CF, Bird RB (1954) Molecular theory of gases and liquids. Wiley, New Jersey
Halgren, T (1996) Merck molecular force field. J Comput Chem 17:490–519
Palmo K, Mirkin NG, Pietila: L, Krimm S (1993) Spectroscopically determined force fields for macromolecules. 1 n-alkane chains. Macromolecules 26:6831–6840
Ren PY, Ponder JW (2003) Polarizable atomic multipole water model for molecular mechanics simulation. J Phys Chem B 107:5933–5947
Lopes PEM, Roux B, MacKerell AD (2009) Molecular modeling and dynamics studies with explicit inclusion of electronic polarizability. Theory and applications. Theor Chem Acc 124:11–28
Khoruzhii O et al (2014) Protein modelling. Springer International Publishing, Basel pp 91–134. https://doi.org/10.1007/978-3-319-09976-7
Shi Y, Ren P, Schnieders M, Piquemal J (2015) Polarizable force fields for biomolecular modeling. In: Parrill AL, Lipkowitz KB (eds) Reviews in computational chemistry. Wiley, New Jersey pp 51–86
Baker CM (2015) Polarizable force fields for molecular dynamics simulations of biomolecules. Wiley Interdiscip Rev Comput Mol Sci 5:241–254
Cieplak P, Dupradeau F-Y, Duan Y, Wang J (2009) Polarization effects in molecular mechanical force fields. J Phys Condens Matter 21:333102
Cisneros GA, Karttunen M, Ren P, Sagui C (2014) Classical electrostatics for biomolecular simulations. Chem Rev 114:779–814
Ji C, Mei Y (2014) Some practical approaches to treating electrostatic polarization of proteins. Acct Chem Res 47:2796–2803
Wang L-P et al (2017) Building a more predictive protein force field: a systematic and reproducible route to AMBER-FB15. J Phys Chem B 121:4023–4039
Cerutti DS, Swope WC, Rice JE, Case DA (2014) ff 14ipq: a self-consistent force field for condensed-phase simulations of proteins. J Chem Theory Comput 10:4515–4534
Hornak V et al (2006) Comparison of multiple amber force fields and development of improved protein backbone parameters. Proteins Struct Funct Bioinf 65:712–725
Debiec KT et al (2016) Further along the road less traveled: AMBER ff15ipq, an original protein force field built on a self-consistent physical model. J Chem Theory Comput 12:3926–3947
Lopes PEM, Guvench O, MacKerell AD (2015) Current status of protein force fields for molecular dynamics simulations. In: Kukol A (ed) Molecular modeling of proteins, methods in molecular biology. Springer, New York, pp 47–71
Takemura K, Kitao A (2012) Water model tuning for improved reproduction of rotational diffusion and NMR spectral density. J Phys Chem B 8:6279–6287
Debiec KT, Gronenborn AM, Chong LT (2014) Evaluating the strength of salt bridges: a comparison of current biomolecular force fields. J Phys Chem B 118:6561–6569
Best RB, Buchete N-V, Hummer G (2008) Are current molecular dynamics force fields too helical? Biophys J 95:L07–L09
Song K, Stewart JM, Fesinmeyer RM, Andersen NH, Simmerling C (2008) Structural insights for designed alanine-rich helices: comparing NMR helicity measures and conformational ensembles from molecular dynamics simulation. Biopolymers 89:747–760
Wang J et al (2012) Development of polarizable models for molecular mechanical calculations. 3. polarizable water models conforming to thole polarization screening schemes. J Phys Chem B 116:7999–8008
Huang J et al (2017) Charmm36M: an improved force field for folded and intrinsically disordered proteins. Nat Methods 14:71–73
Hagler AT (2015) Quantum derivative fitting and biomolecular force fields—functional form, coupling terms, charge flux, nonbond anharmonicity and individual dihedral potentials. J Chem Theory Comput 11:5555–5572
Desgranges C, Delhommelle J (2014) Evaluation of the grand-canonical partition function using expanded Wang-Landau simulations. III. Impact of combining rules on mixtures properties. J Chem Phys 140:104109
Wilson EBJ, Decius JC, Cross PC (1955) Molecular vibrations: the theory of infrared and raman vibrational spectra. McGraw-Bill, New York
Jorgensen WL (1981) Transferable intermolecular potential functions for water, alcohols, and ethers. Application to liquid water. J Am Chem Soc 103:335–340
Wang L-P, Martinez TJ, Pande VS (2014) Building force fields: an automatic, systematic, and reproducible approach. J Phys Chem Lett 5:885–889
Horn HW et al (2004) Development of an improved four-site water model for biomolecular simulations: TIP4P-Ew. J Chem Phys 120:9665–9678
Honda S et al (2008) Crystal structure of a ten-amino acid protein. J Am Chem Soc 130:15327–15331
MacKerell AD, Feig M, Brooks CL (2004) Improved treatment of the protein backbone in empirical force fields. J Am Chem Soc 126:698–699
Wang W, Ye W, Jiang C, Luo R, Chen H (2014) New force field on modeling intrinsically disordered proteins. Chem Biol Drug Des 84:253–269
Perez A, MacCallum JL, Brini E, Simmerling C, Dill KA (2015) Grid-based backbone correction to the ff12SB protein force field for implicit-solvent simulations. J Chem Theory Comput 11:4770–4779
Piana S, Lindorff-Larsen K, Shaw DE (2011) How robust are protein folding simulations with respect to force field parameterization? Biophys J 100:L47–L49
Bernstein J, Hagler AT (1979) Polymorphism and isomorphism as tools to study the relationship between crystal forces and molecular-conformation. Mol Cryst Liq Cryst 50:223–233
Cruz-Cabeza AJ, Bernstein J (2014) Conformational polymorphism. Chem Rev 114:2170–2191
Hagler AT, Moult J, Osguthorpe DJ (1980) Monte-Carlo simulation of the solvent structure in crystals of a hydrated cyclic peptide. Biopolymers 19:395–418
Hall D, Pavitt N (1984) An appraisal of molecular force fields for the representation of polypeptides. J Comput Chem 5:441–450
Kitson DH, Hagler AT (1988) Theoretical-studies of the structure and molecular-dynamics of a peptide crystal. Biochemistry 27:5246–5257
Kitson DH, Hagler AT (1988) Catalysis of a rotational transition in a peptide by crystal forces. Biochemistry 27:7176–7180
Hall D, Pavitt N (1991) Molecular packing and conformational analysis of cyclo- hexaglycyl hemihydrate. J Crystallogr Spectrosc Res 21:241–245
Williams DE (2001) Improved intermolecular force field for molecules containing H, C, N, and O atoms, with application to nucleoside and peptide crystals. J Comp Chem 22:1154–1166
Williams DE (1966) Nonbonbonded potential parameters derived from crystalline aromatic hydrocarbons. J Chem Phys 45:3770–3778
Warshel A, Lifson S (1970) Consistent force field calculations. II. Crystal structures, sublimation energies, molecular and lattice vibrations, molecular conformations, and enthalpies of alkanes. J Chem Phys 53:582–594
Berkovitch-Yellin Z, Ariel S, Leiserowitz L (1983) The comparative roles of the proton-acceptor properties of amide and carboxyl groups in influencing crystal packing patterns: doubly vs. singly hydrogen-bonded systems in N-acyl amino acids and in other amide-acid crystals. J Am Chem Soc 103:765–767
Berkovitch-Yellin Z, Leiserowitz L (1984) The role played by C–H.O and C–H.N interactions in determining molecular packing and conformation. Acta Crystallogr B 40:159–165
Lii JH, Allinger NL, Molecular (1989) Mechanics. The MM3 force field for hydrocarbons. 3. The van der Waals’ potentials and crystal data for aliphatic and aromatic hydrocarbons. J Am Chem Soc 111:8576–8582
Williams DE, Stouch TR (1993) Characterization of force fields for lipid molecules: Applications to crystal structures. J Comput Chem 14:1066–1076
Ewig CS, Thacher TS, Hagler AT (1999) Derivation of class II force fields. 7. Nonbonded force field parameters for organic compounds. J Phys Chem B 103:6998–7014
Donchev AG et al (2007) Assessment of performance of the general purpose polarizable force field QMPFF3 in condensed phase. J Comp Chem 29:1242–1249
Price SL (2014) Predicting crystal structures of organic compounds. Chem Soc Rev 43:2098–2111
Gavezzotti A (2012) Computational studies of crystal structure and bonding. Top Curr Chem 315:1–32
Gavezzotti A (2013) Equilibrium structure and dynamics of organic crystals by Monte Carlo simulation: critical assessment of force fields and comparison with static packing analysis. New J Chem 37:2110–2119
Nyman J, Sheehan Pundyke O, Day GM (2016) Accurate force fields and methods for modelling organic molecular crystals at finite temperatures. Phys Chem Chem Phys 18:15828–15837
Reilly AM et al (2016) Report on the sixth blind test of organic crystal structure prediction methods. Acta Cryst B 72:439–459
Cieplak P, Caldwell J, Kollman P (2001) Molecular mechanical models for organic and biological systems going beyond the atom centered two body additive approximation: aqueous solution free energies of methanol and N-methyl acetamide, nucleic acid base, and amide hydrogen bonding and Chloroform/water partition coefficients of the nucleic acid bases. J Comput Chem 22:1048–1057
Wang J et al (2011) Development of polarizable models for molecular mechanical calculations I: parameterization of atomic polarizability. J Phys Chem B 115:3091–3099
Wang J et al (2011) Development of polarizable models for molecular mechanical calculations II: induced dipole models significantly improve accuracy of intermolecular interaction energies. J Phys Chem B 115:3100–3111
Wang J et al (2012) Development of polarizable models for molecular mechanical calculations. 4. van der Waals parametrization. J Phys Chem B 116:7088–7101
Duan Y et al (2003) A point-charge force field for molecular mechanics simulations of proteins based on condensed-phase quantum mechanical calculations. J Comput Chem 24:1999–2012
Jämbeck JPM, Lyubartsev AP (2014) Update to the general amber force field for small solutes with an emphasis on free energies of hydration. J Phys Chem B 118:3793–3804
Sprenger KG, Jaeger VW, Pfaendtner J (2015) The general AMBER force field (GAFF) can accurately predict thermodynamic and transport properties of many ionic liquids. J Phys Chem B 119:5882–5895
Caldwell JW, Kollman PA (1995) Structure and properties of neat liquids using nonadditive molecular dynamics: water, methanol, and N-methylacetamide. J Phys Chem 99:6208–6219
Wang J, Hou T (2011) Application of molecular dynamics simulations in molecular property prediction. 1. Density and heat of vaporization. J Chem Theory Comput 7:2151–2165
Lifson S (1972) Protein–proptein interactions. Springer, New York pp 3–16
Allinger NL, Chen K, Lii J (1996) An improved force field (MM4) for saturated hydrocarbons. J Comput Chem 17:642–668
Beachy MD, Chasman D, Murphy RB, Halgren TA, Friesner RA (1997) Accurate ab initio quantum chemical determination of the relative energetics of peptide conformations and assessment of empirical force fields. J Am Chem Soc 119:5908–5920
Patel S, MacKerell AD, Brooks CL (2004) CHARMM fluctuating charge force field for proteins: II protein/solvent properties from molecular dynamics simulations using a nonadditive electrostatic model. J Comput Chem 25:1504–1514
Vanommeslaeghe K, MacKerell AD (2015) CHARMM additive and polarizable force fields for biophysics and computer-aided drug design. Biochim Biophys Acta 1850:861–871
Hatcher ER, Guvench O, MacKerell AD (2009) CHARMM additive all-atom force field for acyclic polyalcohols, acyclic carbohydrates, and inositol. J Chem Theory Comput 5:1315–1327
Guvench O et al (2008) Additive empirical force field for hexopyranose monosaccharides. J Comput Chem 29:2543–2564
Mijakovi M et al (2015) A comparison of force fields for ethanol–water mixtures. Mol Simul 699–712:699–712
Guillot B (2002) A reappraisal of what we have learnt during three decades of computer simulations on water. J Mol Liq 101:219–260
Ahlstrom LS, Vorontsov II, Shi J, Miyashita O (2017) Effect of the crystal environment on side-chain conformational dynamics in cyanovirin-N investigated through crystal and solution molecular dynamics simulations. PLoS ONE 12:e0170337
Barone G, Della Gatta G, Ferro D, Piacente V (1990) Enthalpies and entropies of sublimation, vaporization and fusion of nine polyhydric alcohols. J Chem Soc Faraday Trans 86:75–79
He X, Lopes PEM, MacKerell AD (2013) Polarizable empirical force field for acyclic polyalcohols based on the classical Drude oscillator. Biopolymers 99:724–738
Patel DS, He X, Mackerell AD (2015) Polarizable empirical force field for hexopyranose monosaccharides based on the classical Drude oscillator. J Phys Chem B 119:637–652
Clark GNI, Cappa CD, Smith JD, Saykally RJ, Head-Gordon T (2010) The structure of ambient water. Mol Phys 108:1415–1433
Freddolino PL, Park S, Roux B, Schulten K (2009) Force field bias in protein folding simulations. Biophys J 96:3772–3780
Best RB et al (2012) Optimization of the additive CHARMM all-atom protein force field targeting improved sampling of the backbone ϕ, ψ and Side-Chain χ1 and χ2 dihedral angles. J Chem Theory Comput 8:3257–3273
Swaminathan S, Craven BM, Mcmullan RK (1984) The crystal structure and molecular thermal motion of urea at 12, 60 and 123 K from neutron diffraction. Acta Cryst B 40:300–306
Hagler AT, Lifson S (1974) Energy functions for peptides and proteins. II. The amide hydrogen bond and calculation of amide crystal properties. J Am Chem Soc 96:5327–5335
Zaitsau D, Kabo G, Kozyro A, Sevruk V (2003) The effect of the failure of isotropy of a gas in an effusion cell on the vapor pressure and enthalpy of sublimation for alkyl derivatives of carbamide. Thermochim Acta 406:17–28
Piana S, Donchev AG, Robustelli P, Shaw DE (2015) Water dispersion interactions strongly influence simulated structural properties of disordered protein states. J Phys Chem B 119:5113–5123
Rauscher S et al (2015) Structural ensembles of intrinsically disordered proteins depend strongly on force field: a comparison to experiment. J Chem Theory Comput 11:5513–5524
Lindorff-Larsen K et al (2012) Systematic validation of protein force fields against experimental data. PLoS ONE 7:e32131
Li DW, Brüschweiler R (2010) NMR-based protein potentials. Angew Chemie 49:6778–6780
Waldman M, Hagler AT (1993) New combining rules for rare gas van der waals parameters. J Comput Chem 14:1077–1084
Song W, Rossky PJ, Maroncelli M (2003) Modeling alkane + perfluoroalkane interactions using all-atom potentials: failure of the usual combining rules. J Chem Phys 119:9145
Nikitin A, Milchevskiy Y, Lyubartsev A (2015) AMBER-II: new combining rules and force field for perfluoroalkanes. J Phys Chem B 119:14563–14573
Best RB, Hummer G (2009) Optimized molecular dynamics force fields applied to the helix-coil transition of polypeptides. J Phys Chem B 113:9004–9015
Yin J, Henriksen NM, Slochower DR, Gilson MK (2017) The SAMPL5 host–guest challenge: computing binding free energies and enthalpies from explicit solvent simulations by the attach-pull-release (APR) method. J Comput Aided Mol Des 31:133–145
Lindorff-Larsen K et al (2010) Improved side-chain torsion potentials for the Amber ff99SB protein force field. Proteins 78:1950–1958
Kubelka J, Chiu TK, Davies DR, Eaton WA, Hofrichter J (2006) Sub-microsecond protein folding. J Mol Biol 359:546–553
Rohl CA, Baldwin RL (1997) Comparison of NH exchange and circular dichroism as techniques for measuring the parameters of the helix-coil transition in peptides. Biochemistry 36:8435–8442
Izadi S, Anandakrishnan R, Onufriev AV (2014) Building water models: a different approach. J Phys Chem Lett 5:3863–3871
Sullivan MR, Sokkalingam P, Nguyen T, Donahue JP, Gibb BC (2017) Binding of carboxylate and trimethylammonium salts to octa-acid and TEMOA deep-cavity cavitands. J Comput Aided Mol Des 31:21–28
Stouch TR (2012) The errors of our ways: taking account of error in computer-aided drug design to build confidence intervals for our next 25 years. J Comput Aided Mol Des 26:125–134
Chodera JD, Mobley DL (2013) Entropy-enthalpy compensation: Role and ramifications in biomolecular ligand recognition and design. Annu Rev Biophys 2013 42:121–142
Ren P, Wu C, Ponder JW (2011) Polarizable atomic multipole-based molecular mechanics for organic molecules. J Chem Theory Comput 7:3143–3161
Shaik MS, Liem SY, Popelier PLA (2010) Properties of liquid water from a systematic refinement of a high-rank multipolar electrostatic potential. J Chem Phys 132:174504
Morozov AV, Tsemekhman K, Baker D (2006) Electron density redistribution accounts for half the cooperativity of R helix formation. J Phys Chem B 110:4503–4505
Wallqvist A (1990) Incorporating intramolecular degrees of freedom in simulations of polarizable liquid water. Chem Phys 148:439–449
Yin J et al (2017) Overview of the SAMPL5 host–guest challenge: are we doing better? J Comput Aided Mol Des 31:1–19
Lamoureux G, MacKerell AD, Roux B (2003) A simple polarizable model of water based on classical Drude oscillators. J Chem Phys 119:5185
Shi Y et al (2013) Polarizable atomic multipole-based amoeba force field for proteins. J Chem Theory Comput 9:4046–4063
Schmollngruber M, Lesch V, Schrö C, Heuer A, Steinhauser O (2015) Comparing induced point-dipoles and Drude oscillators. Phys Chem Chem Phys 17:14297–14306
Lemkul JA, Huang J, Roux B, MacKerell AD (2016) An empirical polarizable force field based on the classical drude oscillator model: development history and recent applications. Chem Rev 116:4983–5013
Bernal JD, Fowler RH (1933) A theory of water and ionic solution, with particular reference to hydrogen and hydroxyl ions. J Chem Phys 1:515–548
Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML (1983) Comparison of simple potential functions for simulating liquid water. J Chem Phys 79:926–935
Lamoureux G, Harder E, Vorobyov IV, Roux B, MacKerell A (2005) A polarizable model of water for molecular dynamics simulations of biomolecules. Chem Phys Lett 418:2006
Corongiu G, Clementi E (1993) Molecular dynamics simulations with a flexible and polarizable potential: density of states for liquid water at different temperatures. J Chem Phys 98:4984–4990
Dinur U, Hagler AT (1992) The role of nonbond and charge flux in hydrogen bond interactions. The effect on structural changes and spectral shifts in water dimer. J Chem Phys 97:9161–9172
Kitano M, Kuchitsu K (1974) Molecular Structure Of Formamide As Studied By Gas Phase Electron Diffraction. Bull Chem Soc Jpn 47:67–72
Vorobyov IV, Anisimov VM, MacKerell AD (2005) Polarizable empirical force field for alkanes based on the classical Drude oscillator model. J Phys Chem B 109:18988–18999
Steiner T (1996) C–H–O hydrogen bondiong in crystals. Cryst Rev 6:1–57
Bernstein J (2013) ‘It Isn’t’. Cryst Growth Des 13:961–964
Harder E, Anisimov VM, Whitfield T, MacKerell AD, Roux B (2008) Understanding the dielectric properties of liquid amides from a polarizable force field. J Phys Chem B 112:3509–3521
Maple JR, Hwang M, Stockfisch TP, Hagler AT (1994) Derivation of class II force fields. III. Characterization of a quantum force field for alkanes. Isr J Chem 34:195–231
Klauda JB, Brooks BR, MacKerell AD, Venable RM, Pastor RW (2005) An ab initio study on the torsional surface of alkanes and its effect on molecular simulations of alkanes and a DPPC bilayer. J Phys Chem B 109:5300–5311
Shivakumar D, Harder E, Damm W, Friesner RA, Sherman W (2012) Improving the prediction of absolute solvation free energies using the next generation OPLS force field. | Chem Theory Comput 8:2553–2558
Lopes PEM, Lamoureux G, Roux B, MacKerell AD (2007) Polarizable empirical force field for aromatic compounds based on the classical Drude oscillator. J Phys Chem B 111:2873–2885
Hall D, Williams DE (1974) The effect of coulombic interactions on the calculated crystal structures of benzene at atmospheric and 25 kbar pressure. Acta Cryst A 31:56–58
Dzyabchenko AV, Bazilevskii MV (1985) Theoretical structures of crystalline benzene II. Verification of atom–atom potentials. J Struct Chem 26:558–564
Allinger NL, Lii J-H (1987) Benzene, aromatic rings, van der Waals molecules, and crystals of aromatic molecules in molecular mechanics (MM3). J Comput Chem 8:1146–1153
van Eijck BP, Spek aL, Mooij WTM, Kroon J (1998) Hypothetical crystal structures of benzene at 0 and 30 kbar. Acta Crystallogr B 54:291–299
Orabi EA, Lamoureux G (2012) Cation-π and π–π interactions in aqueous solution studied using polarizable potential models. J Chem Theory Comput 8:182–193
Nevins N, Chen K, Allinger NL (1996) Molecular mechanics (MM4) calculations on alkenes. J Comput Chem 17:669–694
Vorobyov I et al (2007) Additive and classical Drude polarizable force fields for linear and cyclic ethers. J Chem Theory Comput 3:1120–1133
Baker CM, MacKerell AD (2010) Polarizability rescaling and atom-based Thole scaling in the CHARMM Drude polarizable force field for ethers. J Mol Model 16:567–576
Anisimov VM, Vorobyov IV, Roux B, MacKerell AD (2007) Polarizable empirical force field for the primary and secondary alcohol series based on the classical Drude model. J Chem Theory Comput 3:1927–1946
Lin B, Lopes PEM, Roux B, MacKerell AD (2013) Kirkwood-Buff analysis of aqueous N-methylacetamide and acetamide solutions modeled by the CHARMM additive and Drude polarizable force fields. J Chem Phys 139:08B624_1
Jorgensen WL, Swenson CJ (1985) Optimized intermolecular potential functions for amides and peptides. Structure and properties of liquid amides. J Am Chem Soc 107:569–578
Wang J, Hou T (2011) Application of molecular dynamics simulations in molecular property prediction II: Diffusion coefficient. J Comput Chem 32:3505–3519
Distasio RA, Jung Y, Head-Gordon MA (2005) Resolution-of-the-identity implementation of the local triatomics-in-molecules model for second-order Møller-Plesset perturbation theory with application to alanine tetrapeptide conformational energies. J Chem Theory Comput 1:862–876
Robertson MJ, Tirado-Rives J, Jorgensen WL (2015) Improved peptide and protein torsional energetics with the OPLS-AA force field. J Chem Theory Comput 11:3499–3509
Lopes PEM et al (2013) Polarizable Force Field for Peptides and Proteins based on the Classical Drude Oscillator. J Chem Theory Comput 9:5430–5449
Li H et al (2015) Representation of ion—protein interactions using the Drude polarizable force-field. J Phys Chem B 8:9401–9416
Ermer O, Lifson S (1973) Consistent force field calculations. III. Vibrations, conformations, and heats of hydrogenation of nonconjugated olefins. J Am Chem Soc 95:4121–4132
Maple JR, Hwang MJ, Jalkanen KJ, Stockfisch TP, Hagler AT (1998) Derivation of class ii force fields: V. Quantum force field for amides, peptides, and related compounds. J Comput Chem 19:430–458
Patel S, Brooks CL (2004) CHARMM fluctuating charge force field for proteins: I parameterization and application to bulk organic liquid simulations. J Comput Chem 25:1–15
Rick SW, Stuart SJ (2002) Potentials and algorithms for incorporating polarizability in computer simulations. Rev Comput Chem 18:89–146
Bauer Ba, Patel S (2012) Recent applications and developments of charge equilibration force fields for modeling dynamical charges in classical molecular dynamics simulations. Theor Chem Acc 131:1153
Rablen PR, Lockman JW, Jorgensen WL (1998) Ab initio study of hydrogen-bonded complexes of small organic molecules with Water. J Phys Chem 102:3782–3797
Rick SW, Stuart SJ, Berne BJ (1994) Dynamical fluctuating charge force fields: application to liquid water. J Chem Phys 101:6141–6156
Zhong Y, Patel S (2010) Nonadditive empirical force fields for short-chain linear alcohols: methanol to butanol. Hydration free energetics and Kirkwood-Buff analysis using charge equilibration models. J Phys Chem B 114:11076–11092
Patel S, Brooks CL III (2005) Structure, thermodynamics, and liquid–vapor equilibrium of ethanol from molecular- dynamics simulations using nonadditive interactions. J Chem Phys 123:164502
Halgren TA (1992) The representation of van der Waals (vdW) interactions in molecular mechanics force fields: potential form, combination rules, and vdW parameters. J Am Chem Soc 114:7827–7843
Ponder JW et al (2010) Current status of the AMOEBA polarizable force field. J Phys Chem B 114:2549–2564
Kumar R, Wang F-F, Jenness GR, Jordan K (2010) A second generation distributed point polarizable water model. J Chem Phys 132:14309
Naserifar S, Brooks DJ, Goddard WA, Cvicek V (2017) Polarizable charge equilibration model for predicting accurate electrostatic interactions in molecules and solids. J Chem Phys 146:124117–124117
Santos LHR, Krawczuk A, Macchi P (2015) Distributed atomic polarizabilities of amino acids and their hydrogen-bonded aggregates. J Phys Chem A 119:3285–3298
Palmo K, Mannfors B, Mirkin NG, Krimm S (2006) Inclusion of charge and polarizability fluxes provides needed physical accuracy in molecular mechanics force fields. Chem Phys Lett 429:628–632
Harder E et al (2016) OPLS3: a force field providing broad coverage of drug-like small molecules and proteins. J Chem Theory Comput 12:281–296
Storer JW, Giesen DJ, Cramer CJ, Truhlar DG (1995) Class IV charge models: a new semiempirical approach in quantum chemistry. J Comput Aided Mol Des 9:87–110
Jakalian A, Jack DB, Bayly CI (2002) Fast, efficient generation of high-quality atomic charges. AM1-BCC model: II. Parameterization and validation. J Comput Chem 23:1623–1641
Distasio RA, Steele RP, Rhee YM, Shao Y, Head-Gordon M (2007) An improved algorithm for analytical gradient evaluation in resolution-of-the-identity second-order Møller-Plesset perturbation theory: application to alanine tetrapeptide conformational analysis. J Comput Chem 28:839–856
Graf J, Nguyen PH, Stock G, Schwalbe H (2007) Structure and dynamics of the homologous series of alanine peptides: a joint molecular dynamics/NMR study. J Am Chem Soc 129:1179–1189
Dodda LS, Vilseck JZ, Tirado-Rives J, Jorgensen WL (2017) 14*CM1A-LBCC: localized bond-charge corrected CM1A charges for condensed-phase simulations. J Phys Chem B 121:3864–3870
Choudhary A, Gandla D, Krow GR, Raines RT (2009) Nature of amide carbonyl–carbonyl interactions in proteins. J Am Chem Soc 131:7244–7246
Wang L et al (2015) Accurate and reliable prediction of relative ligand binding potency in prospective drug discovery by way of a modern free-energy calculation protocol and force field. J Am Chem Soc 137:2695–2703
Maple JR et al (1994) Derivation of class II force fields. 1. Methodology and quantum force-field for the alkyl functional group and alkane molecules. J Comput Chem 15:162–182
Palmo K, Mannfors B, Mirkin NG, Krimm S (2003) Potential energy functions: from consistent force fields to spectroscopically determined polarizable force fields. Biopolymers 68:383–394
Kolǎ M, Hobza P (2012) On extension of the current biomolecular empirical force field for the description of halogen bonds. J Chem Theory Comput 8:1325–1333
Doig AJ, Baldwin RL (1995) N- and C-capping preferences for all 20 amino acids in alpha-helical peptides. Protein Sci 4:1325–1336
Shalongo W, Dugad L, Stellwagen’ E (1994) Distribution of helicity within the model peptide acetyl(AAQAA)3amide. J Am Chem Soc 116:8288–8293
Lifson S, Hagler AT, Dauber P (1979) Consistent force field studies of intermolecular forces in hydrogen-bonded crystals. 1. Carboxylic acids, amides, and the C=O… H–hydrogen bonds. JAm Chem Soc 101:5111
Dunitz JD, Gavezzotti A (2005) Molecular recognition in organic crystals: directed intermolecular bonds or nonlocalized bonding? Angew Chem Int Ed Engl 44:1766–1787
Kazantsev AV et al (2011) Successful prediction of a model pharmaceutical in the fifth blind test of crystal structure prediction. Int J Pharm 418:168–178
Kuhn B et al (2017) Prospective evaluation of free energy calculations for the prioritization of cathepsin L inhibitors. J Med Chem 60:2485–2497
Baliban SM et al (2017) Development of a glycoconjugate vaccine to prevent invasive Salmonella typhimurium infections in sub-Saharan Africa. PLoS Negl Trop Dis 11:1–27
Robinson D et al (2016) Differential water thermodynamics determine PI3K-Beta/Delta selectivity for solvent-exposed ligand modi fi cations. J Chem Inf Model 6:886–894
Dinur U, Hagler AT (1989) Determination of atomic point charges and point dipoles from the Cartesian derivatives of the molecular dipole moment and second moments, and from energy second derivatives of planar dimers II. Applications to model systems. J Chem Phys 91:2959–2970
Dinur U, Hagler AT (1991) New approaches to empirical force fields. In: Lipkowitz KB, Boyd DB (eds) Reviews in Computational Chemistry. VCH Publisher2, New Jersey pp 99–164
Dinur U, Hagler AT (1995) Geometry-dependent atomic charges—methodology and application to alkanes, aldehydes, ketones, and amides. J Comput Chem 16:154–170
Decius JC (1975) An effective atomic charge model for infrared intensities. J Mol Spec 362:348–362
Person WB, Zerbi G (1982) Vibrational intensities in infrared and raman spectroscopy. Elsevier, Amsterdam
Miwa Y, Machida K (1988) Molecular mechanics simulations of thermodynamic functions and infrared spectra of alkanes. J Am Chem Soc 110:5183–5189
Gussoni M, Castiglioni C, Ramos MN, Rui M, Zerbi G (1990) Infrared intensities: from intensity parameters to an overall understanding of the spectrum. J Mol Struc 224:445–470
Stern PS, Chorev M, Goodman M, Hagler AT (1983) Computer-simulation of the conformational properties of retro-inverso peptides. 2. Abinitio study, spatial electron-distribution, and population analysis of N-formylglycine methylamide, N-formyl N’-acetyldiaminomethane, and N-methylmalonamide. Biopolymers 22:1885–1900
Cieplak P, Kollman P (1991) On the use of electrostatic potential derived charges in molecular mechanics force-fields— relative solvation free-energy of Cis-N-methyl-acetamide and trans-N-methyl-acetamide. J Comput Chem 12:1232–1236
Stouch TR, Williams DE (1992) Conformational dependence of electrostatic potential derived charges of a lipid headgroup: glycerylphosphorylcholine. J Comput Chem 13:622–632
Reynolds CA, Essex JW, Richards WG (1992) Atomic charges for variable molecular conformations. J Am Chem Soc 114:9075–9079
Dinur U (1990) ‘Flexible’ water molecules in external electrostatic potentials. J Phys Chem 94:5669–5671
Dinur U, Hagler AT (1989) Direct evaluation of nonbonding interactions from ab initio calculations. J Am Chem Soc 111:5149–5151
Wallqvist A, Ahlstrom P, Karlstroms GA (1990) New intermolecular energy calculation scheme: applications to potential surface and liquid properties of water. J Phys Chem 94:1649–1656
Breneman CM, Wiberg KB (1990) Determining atom-centered monopoles from molecular electrostatic potentials. The need for high sampling density in formamide conformational analysis. J Comput Chem 11:361–373
Hagler AT, Leiserowitz L, Tuval M (1976) Experimental and theoretical studies of barrier to rotation about N-C-Alpha and C-Alpha-C’ bonds (Phi and Psi) in amides and peptides. J Am Chem Soc 98:4600–4612
Wei D, Guo H, Salahub DR (2001) Conformational dynamics of an alanine dipeptide analog: An ab initio molecular dynamics study. Phys Rev E 64:11907
Palmo K, Mirkin NG, Krimm S (1998) Spectroscopically determined force fields for macromolecules. 2. saturated hydrocarbon chains. J Phys Chem A 102:6448–6456
Pietilä L-O (1989) Molecular mechanics and force field calculations in vibrational spectroscopy. J Mol Struct 195:111–132
Palmö K, Pietilä L-O, Krimm S (1991) Construction of molecular mechanics energy functions by mathematical transformation of ab initio force fields and structures. J Comput Chem 12:385–390
Hagler AT, Huler E, Lifson S (1974) Energy functions for peptides and proteins. 1. Derivation of a consistent force-field including the hydrogen-bond from amide crystals. J Am Chem Soc 96:5319–5327
Mannfors B, Palmo K, Krimm S (2008) Spectroscopically determined force field for water dimer: Physically enhanced treatment of hydrogen bonding in molecular mechanics energy functions. J Phys Chem A 112:12667–12678
Mannfors B, Mirkin NG, Palmo K, Krimm S (2001) A polarizable electrostatic model of the N-methylacetamide dimer. J Comput Chem 22:1933–1943
Mannfors B, Palmo K, Krimm S (2000) A new electrostatic model for molecular mechanics force fields. J Mol Struct 556:1–21
Ewig CS, Waldman M, Maple JR (2002) Ab initio atomic polarizability tensors for organic molecules. J Phys Chem A 106:326–334
Albaugh A et al (2016) Advanced potential energy surfaces for molecular simulation. J Phys Chem B. https://doi.org/10.1021/acs.jpcb.6b06414
Kratz EG et al (2016) LICHEM: a QM/MM program for simulations with multipolar and polarizable force fields. J Comput Chem 37:1019–1029
Loboda O, Ingrosso F, Ruiz-López MF, Reis H, Millot C (2016) Dipole and quadrupole polarizabilities of the water molecule as a function of geometry. J Comput Chem 37:2125–2132
Hobza P, Havlas Z (2000) Blue-shifting hydrogen bonds. Chem Rev 100:4253–4264
Honda K (2002) An effective potential function with enhanced charge-transfer-type interaction for hydrogen-bonding liquids. J Chem Phys 117:3558
Řezác J, Hobza P (2016) Benchmark calculations of interaction energies in noncovalent complexes and their applications. Chem Rev 116:5038–5071
Mirkin NG, Krimm S (2014) Note: charge transfer in a hydrated peptide group is determined mainly by its intrinsic hydrogen-bond energetics. J Chem Phys 140:46101
Ren P, Ponder JW (2002) Consistent treatment of inter- and intramolecular polarization in molecular mechanics calculations. J Comp Chem 23:1497–1506
Dudek MJ, Ponder JW (1995) Accurate modeling of the intramolecular electrostatic energy of proteins. J Comput Chem 16:791–816
Rasmussen TD, Ren P, Ponder JW, Jensen F (2007) Force field modeling of conformational energies: importance of multipole moments and intramolecular polarization. Int J Quantum Chem 107:1390–1395
Rowlinson JS (1951) The lattice energy of ice and the second virial coefficient of water vapour. Trans Faraday Soc 47:120–129
Berkovitch-yellin Z, Leiserowitz L (1980) The role of coulomb forces in the crystal packing of amides. A study based on experimental electron densities. J Am Chem Soc 102:7677–7690
Stone AJ (1981) Distributed multipole analysis, or how to describe a molecular charge distribution. Chem Phys Lett 83:233–239
Stone AJ, Price SL (1988) Some new ideas in the theory of intermolecular forces: anisotropic atom–atom. J Phys Chem 92:3325–3335
Price SL (2000) Review in computational chemistry. Wiley, New Jersey pp 225–289
Gresh N, Claverie P, Pullman A (1984) Theoretical studies of molecular conformation. Derivation of an additive procedure for the computation of intramolecular interaction energies. Comparison with ab initio SCF computations. Theor Chim Acta 66:1–20
Gresh N et al (1986) Intermolecular interactions: elaboration on an additive procedure including an explicit charge-transfer contribution. Int J Quantum Chem 24:101–118
Piquemal J-P, Cisneros GA, Reinhardt P, Gresh N, Darden TA (2006) Towards a force field based on density fitting. J Chem Phys 124:104101
Zhang C, Lu C, Wang Q, Ponder JW, Ren P (2015) Polarizable multipole-based force field for dimethyl and trimethyl phosphate. J Chem Theory Comput 11:5326–5339
Thole BT (1981) Molecular polarization calculated with a modified dipole interaction. Chem Phys 59:341–350
Geballe MT, Skillman AG, Nicholls A, Guthrie JP, Taylor PJ (2010) The SAMPL2 blind prediction challenge: introduction and overview. J Comput Aided Mol Des 24:259–279
Gresh N, Kafafi S, Truchon J-F, Salahub DR (2004) Intramolecular interaction energies in model alanine and glycine tetrapeptides. Evaluation of anisotropy, polarization, and correlation effects. A parallel ab initio HF/MP2, DFT, and polarizable molecular mechanics study. J Comput Chem 25:823–834
Shi Y, Schnieders MJ, Ren P (2009) Trypsin-ligand binding free energy calculation with AMOEBA. In: 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, IEEE. pp 2328–2331. https://doi.org/10.1109/IEMBS.2009.5335108
Gresh N, Cisneros GA, Darden T, Piquemal J-P, Anisotropic (2007) Polarizable molecular mechanics studies of inter- and intramolecular interactions and ligand-macromolecule complexes. A bottom–up strategy. J Chem Theory Comput 3:1960–1986
de Courcy B, Piquemal J-P, Garbay C, Gresh N (2010) Polarizable water molecules in ligand—macromolecule recognition. Impact on the relative affinities of competing pyrrolopyrimidine inhibitors for FAK kinase. J Am Chem Soc 132:3312–3320
Zhang J, Yang W, Piquemal JP, Ren P (2012) Modeling structural coordination and ligand binding in zinc proteins with a polarizable potential. J Chem Theory Comput 8:1314–1324
Avbelj F, Moult J, Kitson DH, James MNG, Hagler AT (1990) Molecular-dynamics study of the structure and dynamics of a protein molecule in a crystalline ionic environment, streptomyces-griseus protease-A. Biochemistry 29:8658–8676
Kitson DH et al (1993) On achieving better than 1-Angstrom accuracy in a simulation of a large protein—Streptomyces–Griseus protease-A. Proc Natl Acad Sci USA. 90:8920–8924
Cerutti DS, Freddolino PL, Duke RE Jr, Case DA (2010) Simulations of a protein crystal with a high resolution X-ray structure: evaluation of force fields and water models. J Phys Chem B 114:12811–12824
Cao L et al (2016) Validation of polarizable force field parameters for nucleic acids by inter-molecular interactions. Front Chem Sci Eng 10:203–212
Muddana HS, Fenley AT, Mobley DL, Gilson MK (2014) The SAMPL4 host–guest blind prediction challenge: an overview. J Comput Aided Mol Des 28:305–317
Bell DR et al (2016) Calculating binding free energies of host–guest systems using the AMOEBA polarizable force field. Phys Chem Chem Phys. https://doi.org/10.1039/C6CP02509A
Hirshfeld FL (1977) Bonded-atom fragments for describing molecular charge densities. Theor Chim Acta 44:129–138
Koritsanszky TS, Coppens P (2001) Chemical applications of X-ray charge-density analysis. Chem Rev 101:1583–1627
Halgren TA (1996) Merck Molecular force field. II. MMFF94 van der Waals and electrostatic parameters for intermolecular interactions. J Comput Chem 17:520–552
Bordner AJ, Cavasotto CN, Abagyan RA (2003) Direct derivation of van der Waals force field parameters from quantum mechanical interaction energies. J Phys Chem B 107:9601–9609
Langley CH, Allinger NL (2002) Molecular mechanics (MM4) calculations on amides. J Phys Chem A 106:5638–5652
Uzoh OG, Galek PTA, Price SL (2015) Analysis of the conformational profiles of fenamates shows route towards novel, higher accuracy, force-fields for pharmaceuticals. Phys Chem Chem Phys 17:7936–7948
Perez A, Morrone JA, Simmerling C, Dill K (2016) A. Advances in free-energy-based simulations of protein folding and ligand binding. Curr Opin Struct Biol 36:25–31
Schroeder R, Lippincott ER (1957) Potential function model of hydrogen bonds. J Phys Chem 61:921
Chidambaram R, Balasubramanian R, Ramachandran GN (1970) Potential functions for hydrogen bond interactions I. A modified lippincott-schroeder potential function for NH–O interaction between peptide groups. Biochim Biophys Acta 221:182–195
Momany FA, Carruthers LM, McGuire RF, Scheraga HA (1974) Intermolecular potentials from crystal data. III. Determination of empirical potentials and applications to the packing configurations and lattice energies in crystals of hydrocarbons, carboxylic acids, amines, and amides. J Phys Chem 78:1595–1620
Ewig CS et al (2001) Derivation of class II force fields. VIII. Derivation of a general quantum mechanical force field for organic compounds. J Comput Chem 22:1782–1800
Cerutti DS, Swope WC, Rice JE, Case DA (2014) ff14ipq: A self-consistent force field for condensed-phase simulations of proteins. J Chem Theory Comput 10:4515–4534
Maier JA et al (2015) ff14SB: improving the accuracy of protein side chain and backbone parameters from ff99SB. J Chem Theory Comput 11:3696–3713
Demerdash O, Yap E-H, Head-Gordon T (2014) Advanced potential energy surfaces for condensed phase simulation. Annu Rev Phys Chem 65:149–174
Friesner RA (2005) Modeling polarization in proteins and protein-ligand complexes: methods and preliminary results. Adv Protein Chem 72:79–104
Mohamed NA, Essex JW, Bradshaw RT (2016) Evaluation of solvation free energies for small molecules with the AMOEBA polarizable force field. J Comp Chem 37:2749–2758
Ren P, Wu C, Ponder JW (2012) Polarizable atomic multipole-based molecular mechanics for organic molecules. J Chem Theory Comput 7:3143–3161
Dinur U (1991) Charge flux and electrostatlc forces in planar molecules. J Phys Chem 95:6201–6211
Dinur U, Hagler AT (1990) A novel decomposition of torsional potentials into pairwise interactions—a study of energy 2nd derivatives. J Comput Chem 11:1234–1246
Dinur U, Hagler AT (1994) On the functional representation of bond-energy functions. J Comput Chem 15:919–924
Mannfors B, Sundius T, Palmo K, Pietila L-O, Krimm S (2000) Spectroscopically determined force fields for macromolecules. Part 3. Alkene chains. J Mol Struc 521:49–75
Wang Q et al (2015) A general model for treating short-range electrostatic penetration in a molecular mechanics force field. J Chem Theory Comput 11:2609–2618
Narth C et al (2016) Scalable improvement of SPME multipolar electrostatics in anisotropic polarizable molecular mechanics using a general short-range penetration correction up to quadrupoles. J Comp Chem 37:494–505
Rackers JA et al (2017) An optimized charge penetration model for use with the AMOEBA force field. Phys Chem Chem Phys 19:276–291
Qi R, Wang Q, Ren P (2016) General van der Waals potential for common organic molecules. Bioorg Med Chem. https://doi.org/10.1016/j.bmc.2016.07.062
Ren P, Wu C, Ponder JA (2012) Polarizable atomic multipole-based molecular mechanics for organic molecules. J Chem Theory Comput 7:3143–3161
Galimberti D, Milani A, Castiglioni C (2013) Charge mobility in molecules: charge fluxes from second derivatives of the molecular dipole. J Chem Phys 138:164115
Tafipolsky M, Ansorg K (2016) Toward a physically motivated force field: hydrogen bond directionality from a symmetry-adapted perturbation theory perspective. J Chem Theory Comput 12:1267–1279
Eramian H, Tian Y-H, Fox Z, Beneberu HZ, Kertesz M, Se S (2013) On the anisotropy of van der Waals atomic radii of O. F, Cl, Br, and I. J Phys Chem A 117:14184–14190
Acknowledgements
We would like to thank Drs. Mike Gilson, Sam Krimm, Alex MacKerell, Benoit Roux, Pengyu Ren, Jay Ponder, Ken Dill, Kim Palmo, Pnina Dauber-Osguthorpe, for reading parts of manuscript and helpful discussions and Dr. Ruth Sharon for reading and help with editing. We also thank Eitan Hagler for help with the figures, Martha Obermeier for help with proofing and referee 1 who made many helpful suggestions including the addition of the effect of methodology in FF derivation. Special thanks to the editor, Dr. Terry Stouch for his invitation to write this perspective, encouragement, and endless patience.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hagler, A.T. Force field development phase II: Relaxation of physics-based criteria… or inclusion of more rigorous physics into the representation of molecular energetics. J Comput Aided Mol Des 33, 205–264 (2019). https://doi.org/10.1007/s10822-018-0134-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-018-0134-x
Keywords
- Force fields
- Force field derivation
- Potential functions
- Van der Waals
- Hydrogen bond
- Drug discovery
- Molecular dynamics
- Molecular mechanics
- Protein simulation
- Molecular simulation
- Nonbond interactions
- Combination rules
- Polarizability
- Charge flux
- Nonbond flux
- Polarizability flux
- Free energy
- Coupling terms
- Cross terms
- AMBER
- CHARMM
- OPLS
- GAFF
- AMOEBA
- SDFF
- CFF
- VFF
- Consistent force field
- Electrostatics
- Multipole moments
- Anisotropic nonbond potentials
- Quantum derivative fitting
- QDF