Abstract
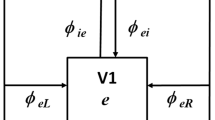
We present a neural field model of binocular rivalry waves in visual cortex. For each eye we consider a one-dimensional network of neurons that respond maximally to a particular feature of the corresponding image such as the orientation of a grating stimulus. Recurrent connections within each one-dimensional network are assumed to be excitatory, whereas connections between the two networks are inhibitory (cross-inhibition). Slow adaptation is incorporated into the model by taking the network connections to exhibit synaptic depression. We derive an analytical expression for the speed of a binocular rivalry wave as a function of various neurophysiological parameters, and show how properties of the wave are consistent with the wave-like propagation of perceptual dominance observed in recent psychophysical experiments. In addition to providing an analytical framework for studying binocular rivalry waves, we show how neural field methods provide insights into the mechanisms underlying the generation of the waves. In particular, we highlight the important role of slow adaptation in providing a “symmetry breaking mechanism” that allows waves to propagate.















Similar content being viewed by others
Notes
From a mathematical perspective, it would also be possible to generate waves when the cross-inhibition is longer range than the excitatory connections, see Fig. 4. Which form is more realistic from the biological perspective depends on which classes of neurons are being taken into account by the neural field model. For example, in visual cortex it is known that excitatory pyramidal cells make both local synaptic contacts as well as longer-range horizontal connections. However, the latter innervate both excitatory and local inhibitory neurons so they could have a net inhibitory effect, thus providing a possible source of long-range inhibition; whether long-range connections generate net excitation or net inhibition also depends on stimulus conditions (Lund et al. 2003).
References
Abbott, L. F., Varela, J. A., Sen, K., & Nelson, S. B. (1997). Synaptic depression and cortical gain control. Science, 275, 220–224.
Amari, S. (1977). Dynamics of pattern formation in lateral-inhibition type neural fields. Biological Cybernetics, 27, 77–87.
Angelucci, A., Levitt, J. B., Walton, E. J. S., Hupe, J.-M., Bullier, J., & Lund, J. S. (2002). Circuits for local and global signal integration in primary visual cortex. Journal of Neuroscience, 22, 8633–8646.
Bart, E., Bao, S., & Holcman, D. (2005). Modeling the spontaneous activity of the auditory cortex.. Journal of Computational Neuroscience, 19, 357–378.
Ben-Yishai, R., Bar-Or, R. L., & Sompolinsky, H. (1995). Theory of orientation tuning in visual cortex. Proceedings of the National Academy of Sciences of the United States of America, 92, 3844–3848.
Blake, R. (2001). A primer on binocular rivalry, including current controversies. Brain and Mind, 2, 5–38.
Blake, R., & Logothetis, N. (2002). Visual competition. Nature Reviews Neuroscience, 3, 1–11.
Blasdel, G. G. (1992). Orientation selectivity, preference, and continuity in monkey striate cortex. Journal of Neuroscience, 12, 3139–3161.
Braun, J. C., & Mattia, M. (2010). Attractors and noise: Twin drivers of decisions and multistability. Neuroimage, 52, 740–751.
Bressloff, P. C., & Cowan, J. D. (2002). An amplitude equation approach to contextual effects in visual cortex. Neural Computation, 14, 493–525.
Bressloff, P. C., Cowan, J. D., Golubitsky, M., Thomas, P. J., & Wiener, M. (2001). Geometric visual hallucinations, Euclidean symmetry and the functional architecture of striate cortex. Philosophical Transactions of the Royal Society B: Biological Sciences, 40, 299–330.
Chance, F. S., Nelson, S. B., & Abbott, L. F. (1998). Synaptic depression and the temporal response characteristics of V1 cells. Journal of Neuroscience, 18, 4785–4799.
Coombes, S., & Owen, M. R. (2004). Evans functions for integral neural field equations with Heaviside firing rate function. SIAM Journal on Applied Dynamical Systems, 3, 574–600.
De Weerd, P., Desimone, R., & Ungerleider, L. G. (1998). Perceptual filling in: A parametric study. Vision Research, 38, 2721–2734.
Ferster, D., & Miller, K. D. (2000). Neural mechanisms of orientation selectivity in the visual cortex. Annual Review of Neuroscience, 23, 441–471.
Fox, R., & Rasche, F. (1969). Binocular rivalry and reciprocal inhibition. Perception & Psychophysics, 5, 215–217.
Gigante, G., Mattia, M., Braun, J., & Del Giudice, P. (2009). Bistable perception modeled as competing stochastic integrations at two levels. PLoS Computational Biological 5(7), e1000430.
Gold, J. M., & Shubel, E. (2006). The spatiotemporal properties of visual completion measured by response classification. Journal of Vision, 6, 356–365.
Hadjikhani, N., Sanchez Del Rio, M., Wu, O., Schwartz, D., Bakker, D., et al. (2001). Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proceedings of the National Academy of Sciences of the United States of America, 98, 4687–4692.
Hubel, D. H., & Wiesel, T. N. (1962). Receptive fields, binocular interaction and functional architecture in the cat’s visual cortex. Journal of Physiology, 160, 106–154.
Hubel, D. H., & Wiesel, T. N. (1977). Functional architecture of macaque monkey visual cortex. Proceedings of the Royal Society of London. Series B, Biological Sciences, 198, 1–59.
Jancke, D., Chavane, F., Maaman, S., & Grinvald, A. (2004). Imaging cortical correlates of illusion in early visual cortex. Nature, 428, 423–426.
Kang, M., Heeger, D. J., & Blake, R. (2009). Periodic perturbations producing phase-locked fluctuations in visual perception. Journal of Vision, 9(2:8), 1–12.
Kang, M., Lee, S.-H., Kim, J., Heeger, D. J., & Blake, R. (2010). Modulation of spatiotemporal dynamics of binocular rivalry by collinear facilitation and pattern-dependent adaptation. Journal of Vision, 10(11:3), 1–15.
Katz, L. C., Gilbert, C. D., & Wiesel, T. N. (1989). Local circuits and ocular dominance columns in monkey striate cortex. Journal of Neuroscience, 9, 1389–1399.
Kilpatrick, Z. P., & Bressloff, P. C. (2010). Binocular rivalry in a competitive neural network with synaptic depression. SIAM Journal of Applied Dynamical Systems, 9, 1303–1347.
Laing, C. R., & Chow, C. C. (2002). A spiking neuron model for binocular rivalry. Journal of Computational Neuroscience, 12, 39–53.
Lee, S.-H., Blake, R., & and Heeger, D. J. (2005). Traveling waves of activity in primary visual cortex during binocular rivalry. Nature Neuroscience, 8, 22–23.
Loxley, P. N., & Robinson, P. A. (2009). Soliton model of competitive neural dynamics during binocular rivalry. Physical Review Letters, 102, 258701.
Lund, J. S., Angelucci, A., & Bressloff, P. C. (2003). Anatomical substrates for functional columns in macaque monkey primary visual cortex. Cerebral Cortex, 12, 15–24.
Moreno-Bote, R., Rinzel, J., & Rubin, N. (2007). Noise-induced alternations in an attractor network model of perceptual bistability. Journal of Neurophysiology, 98, 1125–1139.
Pinto, D. J., & Ermentrout, G. B. (2001). Spatially structured activity in synaptically coupled neuronal networks: I. Traveling fronts and pulses. SIAM Jjournal of Applied Mathematics, 62, 206–228.
Sandstede, B. (2007). Evans functions and nonlinear stability of traveling waves in neuronal network models. International Journal of Bifurcation and Chaos in Applied Sciences and Engineering, 17, 2693–2704.
Shpiro, A., Curtu, R., Rinzel, J., & Rubin, N. (2007). Dynamical characteristics common to neuronal competition models. Journal of Neurophysiology, 97, 462–473.
Shpiro, A., Moreno-Bote, R., Rubin, N. R., & Rinzel, J. (2009). Balance between noise and adaptation in competition models of perceptual bistability. Journal of Computational Neuroscience, 27, 37–54.
Skinner, F. K., Kopell, N., & Marder, E. (1994). Mechanisms for oscillation and frequency control in reciprocally inhibitory model neural networks. Journal of Computational Neuroscience, 1, 69–87.
Sincich, L., & Blasdel, G. G. (2001). Oriented axon projections in primary visual cortex of the monkey. Journal of Neuroscience, 21, 4416–4426.
Sterzer, P., Kleinschmidt, A., & Rees, G. (2009). The neural bases of multistable perception. Trends in Cognitive Sciences, 13, 310–318.
Tabak, J., Senn, W., O’Donovan, M. J., & Rinzel, J. (2000). Modeling of spontaneous activity in developing spinal cord using activity-dependent depression in an excitatory network. Journal of Neuroscience, 20, 3041–3056.
Taylor, A. L., Cottrell, G. W., & Kristan, W. B. (2002). Analysis of oscillations in a reciprocally inhibitory network with synaptic depression. Neural Computation, 14, 561–581.
Tootell, R. B., Switkes, E., Silverman, M. S., & Hamilton, S. L. (1988). Functional anatomy of macaque striate cortex. II. Retinotopic organization. Journal of Neuroscience, 8, 1531–1568.
Tsodyks, M., Pawelzik, K., & Markram, H. (1998). Neural networks with dynamic synapses. Neural Computation, 10, 821–835.
Varela, J. A., Sen, K., Gibson, J., Fost, J., Abbott, L. F., & Nelson, S. B. (1997). A quantitative description of short-term plasticity at excitatory synapses in layer 2/3 of rat primary visual cortex. Journal of Neuroscience, 17, 7926–7940.
Wilson, H. R., Blake, R., & Lee, S. H. (2001). Dynamics of traveling waves in visual perception. Nature, 412, 907–910.
Zhang, L. (2003). On stability of traveling wave solutions in synaptically coupled neuronal networks. Differential and Integral Equations, 16, 513–536.
Author information
Authors and Affiliations
Corresponding author
Additional information
Action Editor: Carson C. Chow
Rights and permissions
About this article
Cite this article
Bressloff, P.C., Webber, M.A. Neural field model of binocular rivalry waves. J Comput Neurosci 32, 233–252 (2012). https://doi.org/10.1007/s10827-011-0351-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-011-0351-y