Abstract
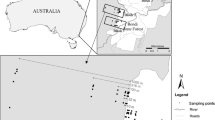
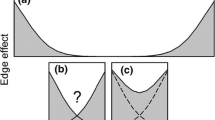
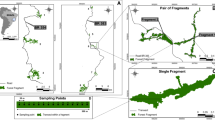
Edge effect is a key process influencing populations and communities, particularly in fragmented landscapes. A general analytical framework has been proposed to quantify the strength of the edge effects (extent and magnitude); however, factors determining the later remain poorly explored. Using a continuous approach we explore the response of dung beetle species and assemblages to ecotones which differ in environmental dissimilarity in the Southern Atlantic forest of Argentina. Using baited pitfall traps and automatic sensors, we estimated dung beetle abundance, microclimatic conditions and vegetation structure along five different forest-plantations transects. At the assemblages level, the majority of species showed either edge avoidance or preference; however, the response depended on the environmental dissimilarity between habitats (plantation and native forest) and varied from a neutral response on mature plantations (low contrast ecotone) to edge avoidance on recent ones (high contrast ecotone). At the species level, the degree of habitat specialization explains the differential response of species to edge effects; more specialized species showed stronger edge response while generalist species showed softer or neutral responses. Environmental dissimilarity between confronted habitats and species specialization explain the quantitative component of edge effects on species and assemblages. Functional groups (rollers and tunnellers) often showed opposite responses to edge effects. At the landscape level, functional connectivity of forest fragments is probably drastically reduced by high contrasts matrices (such as recent plantations) for native forest species, whereas soft ecotones (such as native forest-mature plantations) maintained functional connectivity. These results are particularly relevant on highly fragmented landscapes, such as the Atlantic forest, where edge effect is probably one the most important mechanisms affecting native species and communities.






Similar content being viewed by others
References
Baker SC, Barmuta LA (2006) Evaluation spatial autocorrelation and depletion in pitfall-traps studies of environmental gradients. J Insect Conserv 10:269–276
Banks Leite C, Ewers RM, Metzger JP (2010) Edge effects as the principal cause of area effects on birds in fragmented secondary forest. Oikos 119:918–926
Barragán F, Moreno CE, Escobar F, Halffter G, Navarrete D (2011) Negative impacts of human land use on dung beetle functional diversity. PLoS ONE 6:e17976
Campbell RE, Harding JS, Ewers RM, Thorpe S, Didham RK (2011) Production land use alters edge response functions in remnant forest invertebrate communities. Ecol Appl 21:3147–3161
Colles A, Liow LH, Prinzing A (2009) Are specialists at risk under environmental change? Neoecological, paleoecological and phylogenetic approaches. Ecol Lett 12:849–863
Devictor V, Julliard R, Jiguet F (2008) Distribution of specialist and generalist species along spatial gradients of habitat disturbance and fragmentation. Oikos 117:507–514
Didham RK, Lawton JH (1999) Edge structure determines the magnitude of changes in microclimate and vegetation structure in tropical forest fragments. Biotropica 31:17–30
Ewers RM, Didham RK (2006) Continuous response functions for quantifying the strength of edge effects. J Appl Ecol 43:527–536
Fagan WF, Cantrell RS, Cosner C (1999) How habitat edges change species interactions. Am Nat 153:165–182
Fonseca CR, Joner F (2007) Two sided edge effect studies and the restoration of endangered ecosystems. Restor Ecol 15:613–619
Gardner TA, Hernandez MIM, Barlow J, Peres CA (2008) Understanding the biodiversity consequences of habitat change: the value of secondary and plantation forests for neotropical dung beetles. J Appl Ecol 45:883–893
Hansbauer MM, Storch I, Knauer F, Pilz S, Küchenhoff H, Végvári Z, Pimentel RG, Metzger JP (2010) Landscape perception by forest understory birds in the Atlantic Rainforest: black-and-white versus shades of grey. Land Ecol 25:407–417
Harper KA, Macdonald SE, Burton PJ, Chen J, Brosofske KD, Saunders SC, Euskirchen ES, Roberts DAR, Jaiteh MS, Esseen PERA (2005) Edge influence on forest structure and composition in fragmented landscapes. Conserv Biol 19:768–782
Julliard R, Clavel J, Devictor V, Jiguet F, Couvet D (2006) Spatial segregation of specialists and generalists in bird communities. Ecol Lett 9:1237–1244
Klein BC (1989) Effects of forest fragmentation on dung and carrion beetle communities in central Amazonia. Ecology 70:1715–1725
Koh LP, Lee TM, Sodhi NS, Ghazoul J (2010) An overhaul of the speciesûarea approach for predicting biodiversity loss: incorporating matrix and edge effects. J Appl Ecol 47:1063–1070
Kotze DJ, Lehvävirta S, Koivula M, O’Hara RB, Spence JR (2012) Effects of habitat edges and trampling on the distribution of ground beetles (Coleoptera, Carabaidae) in urban forests. J Insect Conserv. doi:10.1007/s1084101294752
Kraft NJB, Valencia R, Ackerly DD (2008) Functional traits and niche-based tree community assembly in an Amazonian forest. Science 322:580–582
Lacerda ACR, Tomas WM, Marinho-Filho J (2009) Domestic dogs as an edge effect in the Brasília National Park, Brazil: interactions with native mammals. Animal Conserv 12:477–487
Larsen TH, Forsyth A (2005) Trap spacing and transect design for dung beetle biodiversity studies. Biotropica 37:322–325
Lopes J, Korasaki V, Catelli LL, Marcal VVM, Nunes MPBP (2011) A comparison of dung beetle assemblage structure (Coleoptera: Scarabaeidae: Scarabaeinae) between an Atlantic forest fragment and adjacent abandoned pasture in Paraná. Brazil. Zoologia 28:72–79
Murcia C (1995) Edge effects in fragmented forests: implications for conservation. Trends Ecol Evol 10:58–62
Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858
Navarrete D, Halffter G (2008) Dung beetle (Coleoptera: Scarabaeidae: Scarabaeinae) diversity in continuous forest, forest fragments and cattle pastures in a landscape of Chiapas, Mexico: the effects of anthropogenic changes. Biodivers Conserv 17:2869–2898
Nichols E, Larsen T, Spector S, Davis AL, Escobar F, Favila M, Vulinec K (2007) Global dung beetle response to tropical forest modification and fragmentation: a quantitative literature review and meta-analysis. Biol Conserv 137:1–19
Nichols E, Spector S, Louzada J, Larsen T, Amezquita S, Favila ME (2008) Ecological functions and ecosystem services provided by Scarabaeinae dung beetles. Biol Conserv 141:1461–1474
Pawson SM, Brockerhoff EG, Meenken ED, Didham RK (2008) Non-native plantation forests as alternative habitat for native forest beetles in a heavily modified landscape. Biodivers Conserv 17:1127–1148
Pe’er G, Henle K, Dislich C, Frank K (2011) Breaking functional connectivity into components: a novel approach using an individual-based model, and first outcomes. PLoS ONE 6:e22355
Porensky LM (2011) When edges meet: interacting edge effects in an African savanna. J Ecol 99:923–934
Prevedello JA, Figueiredo MSL, Grelle CEV, Vieira MV (2012) Rethinking edge effects: the unaccounted role of geometric constraints. Ecography. doi:10.1111/j.1600-0587.2012.07820.x
Qie L, Lee TM, Sodhi NS, Lim SLH (2010) Dung beetle assemblages on tropical land bridge islands: small island effect and vulnerable species. J Biogeogr 38:792–804
Reino L, Beja P, Osborne PE, Morgado R, Fabipo A, Rotenberry JT (2009) Distance to edges, edge contrast and landscape fragmentation: Interactions affecting farmland birds around forest plantations. Biol Conserv 142:824–838
Ries L, Sisk TD (2004) A predictive model of edge effects. Ecology 85:2917–2926
Ries L, Sisk TD (2010) What is an edge species? The implications of sensitivity to habitat edges. Oikos 119:1636–1642
Ries L, Fletcher RJ Jr, Battin J, Sisk TD (2004) Ecological responses to habitat edges: mechanisms, models, and variability explained. Annu Rev Ecol Evol Syst 35:491–522
Rös M, Escobar F, Halffter G (2012) How dung beetles respond to a human modified variegated landscape in Mexican cloud forest: a study of biodiversity integrating ecological and biogeographical perspectives. Divers Distrib 18:377–389
Scheffler PY (2005) Dung beetle (Coleoptera: Scarabaeidae) diversity and community structure across three disturbance regimes in eastern Amazonia. J Trop Ecol 21:9–19
Silva FAB, Costa CMQ, Moura RC, Farias AI (2010) Study of the dung beetle (Coleoptera: Scarabaeidae) community at two sites: Atlantic Forest and Clear-Cut, Pernambuco, Brazil. Environ Entomol 39:359–367
Simmons LW, Ridsdill-Smith TJ (2011) Ecology and evolution of dung beetles. Wiley-Blackwell, Oxford
Soga M, Kanno N, Yamura Y, Koike S (2012) Patch size determines the strength of edge effects on carabid beetle assemblages in urban remnant forest. J Insect Conserv. doi:10.1007/s108410129524x
Spector S (2006) Scarabaeine dung beetles (Coleoptera: Scarabaeidae: Scarabaeinae): an invertebrate focal taxon for biodiversity research and conservation. Coleopt Bull 60:71–83
Spector S, Ayzama S (2003) Rapid turnover and edge effects in dung beetle assemblages (Scarabaeidae) at a Bolivian neotropical forest savanna ecotone. Biotropica 35:394–404
Van Halder I, Barbaro L, Jactel H (2011) Conserving butterflies in fragmented plantation forests: are edge and interior habitats equally important. J Insect Conserv 15:591–601
Vaz-de-Mello FZ, Edmonds WD, Ocampo FC, Schoolmeesters P (2011) A multilingual key to the genera and subgenera of the subfamily Scarabaeinae of the New World (Coleoptera: Scarabaeidae). Zootaxa 2854:1–73
Vázquez DP, Simberloff D (2002) Ecological specialization and susceptibility to disturbance: conjectures and refutations. Am Nat 159:606–623
Verdú JR, Arellano L, Numa C, Micó E (2007) Roles of endothermy in niche differentiation for ball rolling dung beetles (Coleoptera: Scarabaeidae) along an altitudinal gradient. Ecol Entomol 32:544–551
Wimp GM, Murphy SM, Lewis D, Ries L (2011) Do edge responses cascade up or down a multi-trophic food web? Ecol Lett 14:863–870
Zurita GA, Bellocq MI (2010) Spatial patterns of bird community similarity: bird responses to landscape composition and configuration in the Atlantic forest. Landsc Ecol 25:147–158
Zurita GA, Bellocq MI (2012) Bird assemblages in anthropogenic habitats: identifying a suitability gradient for native species in the Atlantic forest. Biotropica 44:412–419
Zurita GA, Pe’er G, Bellocq MI, Hansbauer MM (2012) Edge effects and their influence on habitat suitability calculations: a continuous approach applied to birds of the Atlantic forest. J Appl Ecol 49:503–512
Acknowledgments
Misiones provincial government (MERNyT of Misiones) and Alto Paraná S.A. gave the appropriate permissions for collecting dung-bettles. This project was funded by the Agencia Nacional de Promoción Científica y Tecnologica (PICT), CONICET and the Universidad de Buenos Aires. Federico Ocampo provided assistance on species identification.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peyras, M., Vespa, N.I., Bellocq, M.I. et al. Quantifying edge effects: the role of habitat contrast and species specialization. J Insect Conserv 17, 807–820 (2013). https://doi.org/10.1007/s10841-013-9563-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-013-9563-y