Abstract
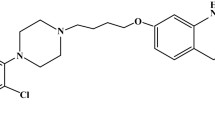
The solubility of risperidone (Risp) in aqueous buffered cyclodextrin (CD) solution was investigated for α-, β-, γ- and HP-β-CD. The effects of pH, ionic strength and temperature on complex stability were also explored. Neutral Risp tends to form higher order complexes (1:2) with both β- and HP-β-CD, but only 1:1 type complexes with α-, and γ-CD. The tendency of Risp to complex with cyclodextrins is in the order β-CD > HP-β-CD > γ-CD > α-CD. The 1:1 complex formation constant of Risp/HP-β-CD increases with increasing ionic strength in an opposite trend to the inherent solubility (S 0) of Risp, thus indicating significant hydrophobic effect. The hydrophobic effect contributes to the extent of 72% towards neutral Risp/HP-β-CD complex stability, while specific interactions contribute only 4.7 kJ/mol. Thermodynamic studies showed that 1:1 Risp/HP-β-CD complex formation is driven by a favorable enthalpy change (ΔH 0=−31.2 kJ/mol, ΔS 0=−7 J/mol.K) while the 1:2 complex is largely driven by entropy changes (ΔH 0=−5.0 kJ/mol, ΔS 0=42 J/mol.K). Complex stability was found to vary with pH, with a higher formation constant for neutral Risp. Molecular mechanical computations using MM (atomic charges and bond dipole algorithms) and Amber force fields, which were carried out to explore possible sites of interactions between Risp and CDs and to rationalize complex stoichiometry, produced similar results concerning optimal inclusion complex geometries and stoichiometries.
Similar content being viewed by others
References
D. Duchene (1987) Cyclodextrins and Their Industrial Uses Editions de Sante’ Paris
J. Szejtli (1998) Chem. Rev. 98 1743 Occurrence Handle10.1021/cr970022c Occurrence Handle11848947
K. Uekama F. Hirayama T. Irie (1998) Chem. Rev. 98 2045 Occurrence Handle10.1021/cr970025p Occurrence Handle11848959
I. Tabushi Y. Kiyousuke T. Sugimoto K. Yamamura (1978) J. Am. Chem. Soc. 100 916 Occurrence Handle10.1021/ja00471a043
M.V. Rekharsky Y. Inoue (1998) Chem. Rev. 98 1875 Occurrence Handle10.1021/cr970015o Occurrence Handle11848952
DJ. Barbirica R.H. Rossib Particlede E.A. Castro (2001) J. Mol. Struct. (THEOCHEM). 537 235 Occurrence Handle10.1016/S0166-1280(00)00680-1
M. Fathallah F. Fotiadu C. Jaime (1994) J. Org. Chem. 59 1288 Occurrence Handle10.1021/jo00085a015
JM. Madrid J. Pozuelo F. Mendicuti W.L. Mattice (1997) J. Coll. Interface. Sci. 193 112 Occurrence Handle10.1006/jcis.1997.5061
JM. Madrid F. Mendicuti W.L. Mattice (1998) J. Phys. Chem. B. 102 2037 Occurrence Handle10.1021/jp9728870
JM. Madrid M. Villafruela R. Serrano F. Mendicuti (1999) J. Phys. Chem. B. 103 4847 Occurrence Handle10.1021/jp9838240
P.M. Ivanov C. Jaime (1996) J. Mol. Struct. 377 137 Occurrence Handle10.1016/0022-2860(95)09133-5
E. Cervello C. Jaime (1998) J. Mol. Struct. (THEOCHEM). 428 195 Occurrence Handle10.1016/S0166-1280(97)00279-0
P. Jiang HW. Sun RX. Shen J. Shi C.M. Lai (2000) J. Mol. Struct. (THEOCHEM). 528 211 Occurrence Handle10.1016/S0166-1280(99)00492-3
A. Mele G. Raffaini F. Ganazzoli M. Juza V. Schurig (2003) Carbohydr. Res. 338 625 Occurrence Handle10.1016/S0008-6215(02)00493-7 Occurrence Handle12644376
M. Faucci F. Melani P. Mura (2002) Chem. Phy. Lett. 358 383 Occurrence Handle10.1016/S0009-2614(02)00410-4
F. Melani N. Mulinacci A. Romani G. Mazzi F.F. Vincieri (1998) Int. J. Pharm. 166 145 Occurrence Handle10.1016/S0378-5173(98)00036-2
E. Cervello F. Mazzucchi C. Jaime (2000) J. Mol. Struct. (THEOCHEM). 530 155 Occurrence Handle10.1016/S0166-1280(00)00328-6
A. Megens (1994) Psychopharmacol. Berl. 114 9
T. Higuchi K.A. Connors (1965) ArticleTitlePhase Solubility Techniques Adv Anal, Chem. Instrum. 4 117
M.B. Zughul A.A. Badwan (1998) J. Inclu. Phenom. Mol. Recog. Chem. 31 243 Occurrence Handle10.1023/A:1007965424219
MB. Zughul M. Al-Omari A.A. Badwan (1998) Pharm. Dev. Technol. 3 43 Occurrence Handle9532599
M.B. Zughul A.A. Badwan (1997) Int. J. Pharm. 151 109 Occurrence Handle10.1016/S0378-5173(97)04901-6
NL. Allinger YH. Yuh J.H. Lii (1989) J. Am. Chem. Soc. 111 8551 Occurrence Handle10.1021/ja00205a001
WD. Cornell P. Cieplak CI. Bayly IR. Gould KM. Merz SuffixJr. DM. Ferguson DC. Spellmeyer T. Fox JW. Caldwell P.A. Kollman (1995) J. Am. Chem. Soc. 117 5179 Occurrence Handle10.1021/ja00124a002
M.J.S. Dewar EG. Zoebisch EF. Healy J.J.P. Stewart (1985) J. Am. Chem. Soc. 107 3902 Occurrence Handle10.1021/ja00299a024
R. Puliti CA. Mattia L. Padiano (1998) Carbohydr. Res. 310 1 Occurrence Handle10.1016/S0008-6215(98)00150-5 Occurrence Handle9867418
K. Linder W. Saenger (1982) Carbohydr. Res. 99 103 Occurrence Handle10.1016/S0008-6215(00)81901-1
W. Saenger J. Jacob K. Gessler T. Steiner D. Hoffman H. Sanbe K. Koizumi S.M. Smith T. Takaha (1998) Chem. Rev. 98 1787 Occurrence Handle10.1021/cr9700181 Occurrence Handle11848949
K. Harata (1987) Bull. Chem. Jpn. 60 2763
A. Yoshida M. Yamamoto T. Itoh T. Irie F. Hirayama K. Uekama (1990) Chem. Pharm. Bull. Tokyo. 38 176 Occurrence Handle2337940
F. Kopecky B. Kopecky P. Kaclik (2001) J. Incl. Phenom. 39 215 Occurrence Handle10.1023/A:1011155208944
A. Buvari-Barcza L. Barcza (1999) Talanta 49 577 Occurrence Handle10.1016/S0039-9140(99)00037-5
A. Buvari-Barcza E. Rak A. Meszaros L. Barcza (1998) J. Incl. Phenom. 32 453 Occurrence Handle10.1023/A:1007989822622
F. D’Anna PL. Meo S. Riela M. Gruttadauria R. Noto (2001) Tetrahedron. 57 6823 Occurrence Handle10.1016/S0040-4020(01)00635-4
SE. Brown JH. Coates PA. Duckworth SF. Lincoln CJ. Easton B.L. May (1993) J. Chem. Soc., Faraday Trans. 89 1035
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El-Barghouthi, M.I., Masoud, N.A., Al-Kafawein, J.K. et al. Host–Guest Interactions of Risperidone with Natural and Modified Cyclodextrins: Phase Solubility, Thermodynamics and Molecular Modeling Studies. J Incl Phenom Macrocycl Chem 53, 15–22 (2005). https://doi.org/10.1007/s10847-004-8212-1
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10847-004-8212-1