Abstract
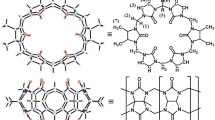
Two new homotritopic guests based on tris(benz)imidazolium salts with adamantane binding sites were prepared. NMR and calorimetric titration experiments revealed that each of the three sites independently binds β-cyclodextrin (β-CD) or cucurbit[7]uril (CB7) units to form binary host–guest complexes with 1:3 stoichiometry. The association constants for the single binding site for β-CD and CB7 were determined using titration calorimetry and are in the order of 105 and 109−10 dm3 mol−1, respectively. In addition, both guests were able to form ternary systems with β-CD and CB7 in ratios of 1:1:2 and 1:2:1, respectively.







Similar content being viewed by others
References
Xing, M., Yanli, Z.: Biomedical applications of supramolecular systems based on host–guest interactions. Chem. Rev. 115, 7794–7839 (2015)
Li, M., Zhou, C., Quanzhu, Y., Xiaogang, Y., Chao, Z., Liqiong, L.: Polymeric supramolecular materials and their biomedical applications. Curr. Org. Chem. 18, 1937–1947 (2014)
Wang, D., Tong, G., Dong, R., Zhou, Y., Shen, J., Zhu, X.: Self-assembly of supramolecularly engineered polymers and their biomedical applications. Chem. Commun. 50, 11994–12017 (2014)
Dong, R., Zhou, Y., Zhu, X.: Supramolecular dendritic polymers: from synthesis to applications. Acc. Chem. Res. 47, 2006–2016 (2014)
Hu, J., Liu, S.: Engineering responsive polymer building blocks with host–guest molecular recognition for functional applications. Acc. Chem. Res. 47, 2084–2095 (2014)
Zhang, J., Ma, P.X.: Cyclodextrin-based supramolecular systems for drug delivery: recent progress and future perspective. Adv. Drug. Deliv. Rev. 65, 1215–1233 (2013)
Kejik, Z., Kaplanek, R., Briza, T., Kralova, J., Martasek, P., Kral, V.: Supramolecular approach for target transport of photodynamic anticancer agents. Supramol. Chem. 24, 106–116 (2012)
De Greef, T.F.A., Smulders, M.M.J., Wolffs, M., Schenning, A.P.H.J., Sijbesma, R.P., Meijer, E.W.: Supramolecular polymerization. Chem. Rev. 109, 5687–5754 (2009)
Dong, S., Bo, Z., Wang, F., Huang, F.: Supramolecular polymers constructed from macrocycle-based host–guest molecular recognition motifs. Acc. Chem. Res. 47, 1982–1994 (2014)
Charlot, A., Velty, R.A.: Novel hyaluronan-based supramolecular assemblies stabilized by multivalent specific interactions: rheological behavior in aqueous solution. Macromolecules 40, 9555–9563 (2007)
Layre, A.M., Volet, G., Wintgens, V., Amiel, C.: Associative network based on cyclodextrin polymer: a model system for drug delivery. Biomacromolecules 10, 3283–3289 (2009)
Li, L., Guo, X., Wang, J., Liu, P., Prud’homme, R.K., May, B.L., Lincoln, S.F.: Polymer networks assembled by host—guest inclusion between adamantyl and β-cyclodextrin substituents on poly(acrylic acid) in aqueous solution. Macromolecules 41, 8677–8681 (2008)
Heyden, A.V., Wilczewski, M., Labbe, P., Auzely, R.: Multilayer films based on host–guest interactions between biocompatible polymers. Chem. Commun. 3220–3222 (2006)
Charlot, A., Velty, R.A.: Synthesis of novel supramolecular assemblies based on hyaluronic acid derivatives bearing bivalent β-cyclodextrin and adamantane moieties. Macromolecules 40, 1147–1158 (2007)
Li, C., Luo, G.F., Wang, H.Y., Zhang, J., Gong, Y.H., Cheng, S.X.: Host–guest assembly of pH-responsive degradable microcapsules with controlled drug release behavior. J. Phys. Chem. 115, 17651–17659 (2011)
Bistri, O., Mazeau, K., Velty, R.A., Sollogoub, M.: A hydrophilic cyclodextrin duplex forming supramolecular assemblies by physical cross-linking of a biopolymer. Chem. Eur. J. 13, 8847–8857 (2007)
Nally, R., Isaacs, L.: Toward supramolecular polymers incorporating double cavity cucurbituril hosts. Tetrahedron 65, 7249–7258 (2009)
Liu, Y., Fang, R., Tan, X., Wang, Z., Zhang, X.: Supramolecular polymerization at low monomer concentrations: enhancing intermolecular interactions and suppressing cyclization by rational molecular design. Chem. Eur. J. 18, 15650–15654 (2012)
Galantini, L., Jover, A., Leggio, C., Meijide, F., Pavel, N.V., Tellini, V.H.S., Tato, J.V., Tortolini, C.: Early stages of formation of branched host−guest supramolecular polymers. J. Phys. Chem. B 112, 8536–8541 (2008)
Leggio, C., Anselmi, M., Nola, A.D., Galantini, L., Jover, A., Meijide, F., Pavel, N.V., Tellini, V.H.S., Tato, J.V.: Study on the structure of host–guest supramolecular polymers. Macromolecules 40, 5899–5906 (2007)
Hasegawa, Y., Miyauchi, M., Takashima, Y., Harada, A.: Supramolecular polymers formed from β-cyclodextrins dimer linked by poly (ethylene glycol) and guest dimers. Macromolecules 38, 3724–3730 (2005)
Krishnan, R., Gopidas, K.: β-Cyclodextrin as an end-to-end connector. J. Phys. Chem. Lett. 2, 2094–2098 (2011)
Ohga, K., Takashima, Y., Takahashi, H., Kawaguchi, Y., Yamaguchi, H., Harada, A.: Preparation of supramolecular polymers from a cyclodextrin dimer and ditopic guest molecules: control of structure by linker flexibility. Macromolecules 38, 5897–5904 (2005)
Miyawaki, A., Takashima, Y., Yamaguchi, H., Harada, A.: Branched supramolecular polymers formed by bifunctional cyclodextrin derivatives. Tetrahedron 64, 8355–8361 (2008)
Bohm, I., Isenbugel, K., Ritter, H., Branscheid, R., Kolb, U.: Cyclodextrin and adamantane host-guest interactions of modified hyperbranched poly(ethylene imine) as mimetics for biological membranes. Angew. Chem. Int. Ed. 50, 7896–7899 (2011)
Schmidt, B.V.K.J., Rudolph, T., Hetzer, M., Ritter, H., Schacher, F.H., Barner-Kowollik, C.: Supramolecular three-armed star polymers via cyclodextrin host–guest self-assembly. Polym. Chem. 3, 3139–3145 (2012)
Bednaříková, T., Tošner, Z., Horský, J., Jindřich, J.: Synthesis of C 3 -symmetric tri(alkylamino) guests and their interaction with cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 81, 141–152 (2015)
Takashima, Y., Yuting, Y., Otsubo, M., Yamaguchi, H., Harada, A.: Supramolecular hydrogels formed from poly(viologen) cross-linked with cyclodextrin dimers and their physical properties. Beilstein J. Org. Chem. 8, 1594–1600 (2012)
Osman, S.K., Brandl, F.P., Zayed, G.M., Tebmer, J.K., Gopferich, A.M.: Cyclodextrin based hydrogels: inclusion complex formation and micellization of adamantane and cholesterol grafted polymers. Polymer 52, 4806–4812 (2011)
Koopmans, C., Ritter, H.: Formation of physical hydrogels via host–guest interactions of β-cyclodextrin polymers and copolymers bearing adamantyl groups. Macromolecules 41, 7418–7422 (2008)
Crini, G.: Review: a history of cyclodextrins. Hist. Cyclodext. Chem. Rev. 114, 10940–10975 (2014)
Del Valle, E.M.M.: Cyclodextrins and their uses: a review. Process Biochem. 39, 1033–1046 (2004)
Kraus, T.: Modified cyclodextrins with pendant cationic and anionic moieties as hosts for highly stable inclusion complexes and molecular recognition. Curr. Org. Chem. 15, 802–814 (2011)
Bricout, H., Hapiot, F., Ponchel, A., Tilloy, S., Monflier, F.: Chemically modified cyclodextrins: an attractive class of supramolecular hosts for the development of aqueous biphasic catalytic processes. Sustainability 1, 924–945 (2009)
Brewster, M.E., Loftsson, T.: The use of chemically modified cyclodextrins in the development of formulations for chemical delivery systems. Pharmazie 57, 94–101 (2002)
Hattori, K., Ikeda, H.: Modification reactions of cyclodextrins and the chemistry of modified cyclodextrins. In: Dodziuk, H. (ed.) Cyclodextrins and their complexes, pp. 31–64. Wiley-VCH, Weinheim (2006)
Isaacs, L.: Cucurbit[n]urils: from mechanism to structure and function. Chem. Commun. 619–629 (2009)
Isaacs, L.: Stimuli responsive systems constructed using cucurbit[n]uril-type molecular containers. Acc. Chem. Res. 47, 2053–2062 (2014)
Kaifer, A.E.: Toward reversible control of cucurbit[n]uril complexes. Acc. Chem. Res. 47, 2160–2167 (2014)
Rekharsky, M.V., Mori, T., Yang, C., Ko, Y.H., Selvapalam, N., Kim, H., Sobransingh, D., Kaifer, A.E., Liu, S., Isaacs, L., Chen, W., Moghaddam, S., Gilson, M.K., Kim, K., Inoue, Y.: A synthetic host–guest system achieves avidin-biotin affinity by overcoming enthalpy–entropy compensation. Proc. Natl. Acad Sci. USA 104, 20737–20742 (2007)
Moghaddam, S., Yang, C., Rekharsky, M.V., Ko, Y.H., Kim, K., Inoue, Y., Gilson, M.K.: New ultrahigh affinity host–guest complexes of cucurbit[7]uril with bicyclo[2.2.2]octane and adamantane guests: thermodynamic analysis and evaluation of M2 affinity calculations. J. Am. Chem. Soc. 133, 3570–3581 (2011)
Cao, L., Šekutor, M., Zavalij, P.Y., Mlinarić-Majerski, K., Glaser, R., Isaacs, L.: Cucurbit[7]uril·guest pair with an attomolar dissociation constant. Angew. Chem. Int. Ed. 53, 988–993 (2014)
Agrigento, P., Al-Amsyar, S.M., Soree, B., Taherimehr, M., Gruttadauria, M., Aprile, C., Pescarmona, P.P.: Synthesis and high-throughput testing of multilayered supported ionic liquid catalysts for the conversion of CO2 and epoxides into cyclic carbonates. Catal. Sci. Tech. 4, 1598–1607 (2014)
Ghosh, D., Lee, J.Y., Liu, C.Y., Chiang, Y.H., Lee, H.M.: Direct C–H arylations of unactivated arenes catalyzed by amido-functionalized imidazolium salts. Adv. Synth. Catal. 356, 406–410 (2014)
Byeun, A., Baek, K., Han, M.S., Lee, S.: Palladium-catalyzed C–S bond formation by using N-amido imidazolium salts as ligands. Tetrahedron Lett. 54, 6712–6715 (2013)
Myles, L., Gore, R.G., Gathergood, N., Connon, S.J.: A new generation of aprotic yet Brønsted acidic imidazolium salts: low toxicity, high recyclability and greatly improved activity. Green Chem. 15, 2740–2746 (2013)
Li, H., Chen, J., Hua, L., Qiao, Y., Yu, Y., Pan, Z., Yang, H., Hou, Z.: Polyoxometalate and copolymer-functionalized ionic liquid catalyst for esterification. Pure Appl. Chem. 84, 541–551 (2012)
Wang, Y., Robinson, G.H.: N-Heterocyclic carbene—main-group chemistry: a rapidly evolving field. Inorg. Chem. 53, 11815–11832 (2014)
Hopkinson, M.N., Richter, C., Schedler, M., Glorius, F.: An overview N-heterocyclic carbenes. Nature 510, 485–496 (2014)
Riener, K., Haslinger, S., Raba, A., Hogerl, M.P., Cokoja, M., Herrmann, W.A., Kuhn, F.E.: Chemistry of iron N-heterocyclic carbene complexes: syntheses, structures, reactivities, and catalytic applications. Chem. Rev. 114, 5215–5272 (2014)
Chauhan, P., Enders, D.: N-Heterocyclic carbene catalyzed activation of esters: a new option for asymmetric domino reactions. Angew. Chem. Int. Ed. 53, 1485–1487 (2014)
Ranganath, K.V.S., Onitsuka, S., Kumar, A.K., Inanaga, J.: Recent progress of N-heterocyclic carbenes in heterogeneous catalysis. Catal. Sci. Tech. 3, 2161–2181 (2013)
Rovis, T., Nolan, S.P.: Stable carbenes: from ‘laboratory curiosities’ to catalysis mainstays. Synlett 24, 1188–1189 (2013)
Černochová, J., Branná, P., Rouchal, M., Kulhánek, P., Kuřitka, I., Vícha, R.: Determination of intrinsic binding modes by mass spectrometry: gas-phase behavior of adamantylated bisimidazolium guests complexed to cucurbiturils. Chem. Eur. J. 18, 13633–13637 (2012)
Zhao, N., Liu, L., Biedermann, F., Scherman, O.A.: Binding studies on CB[6] with a series of 1-alkyl-3-methylimidazolium ionic liquids in an aqueous system. Chem. Asian. J. 5, 530–537 (2010)
Schneider, H.-J., Hacket, F., Rüdiger, V.: NMR studies of cyclodextrins and cyclodextrin complexes. Chem. Rev. 98, 1755–1785 (1998)
Patiny, L., Borel, A.: ChemCalc: a building block for tomorrow’s chemical infrastructure. J. Chem. Inf. Model. 53, 1223–1228 (2013)
Acknowledgments
This work was supported by the Internal Funding Agency of the Tomas Bata University in Zlín under Grant IGA/FT/2015/005.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kulkarni, S.G., Prucková, Z., Rouchal, M. et al. Adamantylated trisimidazolium-based tritopic guests and their binding properties towards cucurbit[7]uril and β-cyclodextrin. J Incl Phenom Macrocycl Chem 84, 11–20 (2016). https://doi.org/10.1007/s10847-015-0577-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-015-0577-9