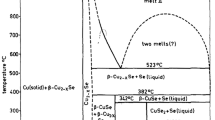
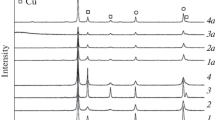
Abstract
Covellite, CuS and chalcocite, Cu2S nanoparticles prepared in the explosive manner from elemental precursors were further ball-milled in order to observe additional changes caused by mechanical action. Three phases of chalcocite were interchanging during milling, monoclinic one being major at the equilibrium after 30 min. In the case of covellite synthesis, milling for 15 min brought about a significant diminishment in the content of digenite, Cu1.8S, impurity. Covellite powder exhibited finer character than chalcocite, as documented by crystallite size, grain size and specific surface area analysis. Finally, the effect of milling speed on the explosive character of the reaction and phase composition of chalcocite was investigated. The most drastic conditions favored the formation of the monoclinic phase with the lowest symmetry and the time and intensity of the explosion was found to depend on the milling speed. The whole process is mechanically driven.













Similar content being viewed by others
References
Roy P, Srivastava SK (2015) Nanostructured copper sulfides: synthesis, properties and applications. CrystEngComm 17:7801
Shamraiz U, Hussain RA, Badshah A (2016) Fabrication and applications of copper sulfide (CuS) nanostructures. J Solid State Chem 238:25
Goel S, Chen F, Cai WB (2014) Synthesis and biomedical applications of copper sulfide nanoparticles: from sensors to theranostics. Small 10:631
Xiao ZY (2014) CuS nanoparticles: clinically favorable materials for photothermal applications? Nanomedicine 9:373
Vaughan DJ, Craig JR (1978) Mineral chemistry of metal sulfides. Cambridge University Press, Cambridge
Sabah FA, Ahmed NM, Hassan Z, Rasheed HS (2016) High performance CuS p-type thin film as a hydrogen gas sensor. Sens Actuators A Phys 249:68
Cuevas A, Romero R, Dalchiele EA, Ramos-Barrado JR, Martin F, Leinen D (2016) Spectrally selective CuS solar absorber coatings on stainless steel and aluminum. Surf Interface Anal 48:649
Mulla R, Rabinal MK (2017) Ambient growth of highly oriented Cu2S dendrites of superior thermoelectric behaviour. Appl Surf Sci 397:70
Farhadi S, Siadatnasab F (2016) Copper(I) sulfide (Cu2S) nanoparticles from Cu(II) diethyldithiocarbamate: synthesis, characterization and its application in ultrasound-assisted catalytic degradation of organic dye pollutants. Mater Res Bull 83:345
Zhao W, Wang ZH, Zhou L, Liu NQ, Wang HX (2016) Natural sunlight irradiated flower-like CuS synthesized from DMF solvothermal treatment. Front Mater Sci 10:290
Xie Y, Carbone L, Nobile C et al (2013) Metallic-like stoichiometric copper sulfide nanocrystals: phase- and shape-selective synthesis, near-infrared surface plasmon resonance properties, and their modeling. ACS Nano 7:7352
Jen-La Plante I, Zeid TW, Yang PD, Mokari T (2010) Synthesis of metal sulfide nanomaterials via thermal decomposition of single-source precursors. J Mater Chem 20:6612
Nafees M, Ali S, Rasheed K, Idrees S (2012) The novel and economical way to synthesize CuS nanomaterial of different morphologies by aqueous medium employing microwaves irradiation. Appl Nanosci 2:157
Kristl M, Ban I, Gyergyek S (2013) Preparation of nanosized copper and cadmium chalcogenides by mechanochemical synthesis. Mater Manuf Process 28:1009
Yang DW, Su XL, Yan YG, He J, Uher C, Tang XF (2016) Mechanochemical synthesis of high thermoelectric performance bulk Cu2X (X = S, Se) materials. APL Mater 4:116110
Baláž P, Achimovičová M, Baláž M et al (2013) Hallmarks of mechanochemistry: from nanoparticles to technology. Chem Soc Rev 42:7571
Blachnik R, Muller A (2000) The formation of Cu2S from the elements I. Copper used in form of powders. Thermochim Acta 361:31
Ou Z, Li J (2014) Synergism of mechanical activation and sulfurization to recover copper from waste printed circuit boards. RSC Adv 4:51970
Li S, Ge ZH, Zhang BP et al (2016) Mechanochemically synthesized sub-5 nm sized CuS quantum dots with high visible-light-driven photocatalytic activity. Appl Surf Sci 384:272
Wang XB, Xu CQ, Zhang ZC (2006) Synthesis of CuS nanorods by one-step reaction. Mater Lett 60:345
Tolia JV, Chakraborty M, Murthy ZVP (2012) Mechanochemical synthesis and characterization of group II–VI semiconductor nanoparticles. Part Sci Technol 30:533
Liu LG, Liu C, Fu WP, Deng LG, Zhong HZ (2016) Phase transformations of copper sulfide nanocrystals: towards highly efficient quantum-dot-sensitized solar cells. ChemPhysChem 17:771
Jiang XC, Xie Y, Lu J, He W, Zhu LY, Qian YT (2000) Preparation and phase transformation of nanocrystalline copper sulfides (Cu9S8, Cu7S4 and CuS) at low temperature. J Mater Chem 10:2193
Lin IJ, Nadiv S (1979) Review of the phase transformation and synthesis of inorganic solids obtained by mechanical treatment (mechanochemical reactions). Mater Sci Eng 39:193
Bakker H, Zhou GF, Yang H (1995) Mechanically driven disorder and phase-transformations in alloys. Prog Mater Sci 39:159
Šepelák V, Bégin-Colin S, Le Caer G (2012) Transformations in oxides induced by high-energy ball-milling. Dalton Trans 41:11927
Ipus JJ, Blázquez JS, Franco V et al (2008) An equivalent time approach for scaling the mechanical alloying processes. Intermetallics 16:470
Abdellaoui M, Gaffet E (1995) The physics of mechanical alloying in a planetary ball mill—mathematical treatment. Acta Metall Mater 43:1087
Magini M, Iasonna A, Padella F (1996) Ball milling: an experimental support to the energy transfer evaluated by the collision model. Scr Mater 34:13
Gotor FJ, Achimovičová M, Real C, Baláž P (2013) Influence of the milling parameters on the mechanical work intensity in planetary mills. Powder Technol 233:1
Baláž M, Zorkovská A, Urakaev F et al (2016) Ultrafast mechanochemical synthesis of copper sulfides. RSC Adv 6:87836
Takacs L (2002) Self-sustaining reactions induced by ball milling. Prog Mater Sci 47:355
Urakaev FK (2013) Experimental study of mechanically induced self-propagating reactions in metal-sulfur mixtures. Combust Sci Technol 185:473
Leon M, Terao N, Rueda F (1984) Phase transitions in cuprous sulphide evaporated thin films. J Mater Sci 19:113. doi:10.1007/BF00552999
Gronvold F, Westrum EF (1987) Thermodynamics of copper sulfides I. Heat-capacity and thermodynamic properties of copper(I) sulfide, Cu2S, from 5 to 950 K. J Chem Thermodyn 19:1183
Wang K, Tan GL (2010) Synthesis and optical properties of CuS nanocrystals by mechanical alloying process. Curr Nanosci 6:163
Heydari H, Moosavifard SE, Elyasi S, Shahraki M (2017) Nanoporous CuS nano-hollow spheres as advanced material for high-performance supercapacitors. Appl Surf Sci 394:425
Opoczky L (1977) Fine grinding and agglomeration of silicates. Powder Technol 17:1
Juhász AZ (1990) Mechanical activation of minerals by grinding: pulverizing and morphology of particles. Ellis Horwood, Chichester
Achimovičová M, Daneu N, Dutková E, Zorkovská A (2017) Mechanochemically synthesized cobalt monoselenide: structural characterization and optical properties. Appl Phys A 123:154
Baláž P, Baláž M, Shpotyuk O et al (2017) Properties of arsenic sulphide (β-As4S4) modified by mechanical activation. J Mater Sci 52:1747. doi:10.1007/s10853-016-0466-7
Gheisari K, Javadpour S, Oh JT, Ghaffari M (2009) The effect of milling speed on the structural properties of mechanically alloyed Fe–45%Ni powders. J Alloys Compd 472:416
Trapp J, Kieback B (2013) Solid-state reactions during high-energy milling of mixed powders. Acta Mater 61:310
Urakaev FK (2010) Mechanism and kinetics of mechanochemical processes. In: High-energy ball milling: mechanochemical processing of nanopowders, p 9
Urakaev FK, Boldyrev VV (2000) Mechanism and kinetics of mechanochemical processes in comminuting devices 2. Applications of the theory. Experiment. Powder Technol 107:197
Urakaev FK, Boldyrev VV (2000) Mechanism and kinetics of mechanochemical processes in comminuting devices—1. Theory. Powder Technol 107:93
Acknowledgements
The present work was financially supported by the Slovak Research and Development Agency under the contract No. APVV-14-0103 and by Slovak Grant Agency VEGA (project 2/0027/14). Spanish Ministry of Science and Innovation and EU FEDER (MAT-2013-45,165-P) and MAT-2016-77265-R (AEI/FEDER, UE) are also acknowledged. The support of German Federal Ministry of Education and Research (project IB-COMSTRUC-010) is also appreciated. The authors also acknowledge the financial support from the Slovenian Research Agency (research core funding No. P2-0084).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Baláž, M., Zorkovská, A., Blazquez, J.S. et al. Mechanochemistry of copper sulphides: phase interchanges during milling. J Mater Sci 52, 11947–11961 (2017). https://doi.org/10.1007/s10853-017-1189-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-1189-0