Abstract
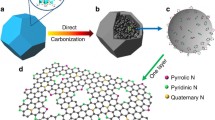
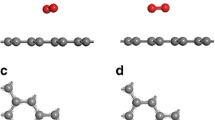
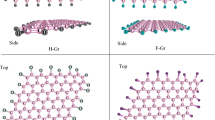
Nitrogen-doped carbon nanomaterials have greater capacity and better cycling stability for Li-ion batteries as compared to undoped carbon materials. In situ near-edge X-ray absorption fine structure (NEXAFS) spectroscopy in combination with quantum chemical modeling has been applied to determine the chemical states of the incorporated nitrogen after interaction with lithium. NEXAFS N K-edge spectra of nitrogen-doped porous carbon were measured before and after thermal deposition of Li vapors. The simulation and interpretation of NEXAFS data were carried out based on density functional theory calculations of initial and lithiated graphene fragments that contained different nitrogen species. The preferable interactions of Li with pyridinic and hydrogenated pyridinic nitrogen which are located at edges of atomic vacancies and graphene planes were revealed.





Similar content being viewed by others
References
Lahiri I, Choi W (2013) Carbon nanostructures in lithium ion batteries: past, present, and future. Crit Rev Solid State Mater Sci 38:128–166
Rahman MA, Wong YC, Song G, Wen C (2015) A review on porous negative electrodes for high performance lithium-ion batteries. J Porous Mater 22:1313–1343
Zhou Z, Zhang H, Zhou Y, Qiao H, Gurung A, Naderi R, Elbohy H, Smirnova AL, Lu H, Chen S, Qiao Q (2017) Binder free hierarchical mesoporous carbon foam for high performance lithium ion battery. Sci Rep 7:1440. https://doi.org/10.1038/s41598-017-01638-y
Zhao D, Wang L, Yu P, Zhao L, Tian C, Zhou W, Zhang L, Fu H (2015) From graphite to porous graphene-like nanosheets for high rate lithium-ion batteries. Nano Res 8(9):2998–3010
Zhou H, Zhu S, Hibino M, Honma I, Ichihara M (2003) Lithium storage in ordered mesoporous carbon (CMK-3) with high reversible specific energy capacity and good cycling performance. Adv Mater 15:2107–2111
Bokhari SW, Siddique AH, Pan H, Li Y, Imtiaz M, Chen Z, Zhu SM, Zhang D (2017) Nitrogen doping in the carbon matrix for Li-ion hybrid supercapacitors: state of the art, challenges and future prospective. RSC Adv 7:18926–18936
Wang Z-L, Xu D, Wang H-G, Wu Z, Zhang X-B (2013) In situ fabrication of porous graphene electrodes for high-performance energy storage. ACS Nano 7(3):2422–2430
Li X, Geng D, Zhang Y, Meng X, Li R, Sun X (2011) Superior cycle stability of nitrogen-doped graphene nanosheets as anodes for lithium ion batteries. Electrochem Commun 13:822–825
Wu Z-S, Ren W, Xu L, Li F, Cheng H-M (2011) Doped graphene sheets as anode materials with superhigh rate and large capacity for lithium ion batteries. ACS Nano 5:5463–5471
Bulusheva LG, Okotrub AV, Kurenya AG, Zhang H, Zhang H, Chen X, Song H (2011) Electrochemical properties of nitrogen-doped carbon nanotube anode in Li-ion batteries. Carbon 49:4013–4023
Reddy ALM, Srivastava A, Gowda SR, Gullapalli H, Dubey M, Ajayan PM (2010) Synthesis of nitrogen-doped graphene films for lithium battery application. ACS Nano 4:6337–6342
Wang X, Weng Q, Liu X, Wang X, Tang D-M, Tian W, Zhang C, Liu WYD, Bando Y, Golberg D (2014) Atomistic origins of high rate capability and capacity of N-doped graphene for lithium storage. Nano Lett 14:1164–1171
Tian L-L, Wei X-Y, Zhuang Q-C, Jiang C-H, Wu C, Ma G-Y, Zhao X, Zonga Z-M, Sunc S-G (2014) Bottom-up synthesis of nitrogen-doped graphene sheets for ultrafast lithium storage. Nanoscale 6:6075–6083
Yao F, Günes F, Ta HQ, Lee SM, Chae SJ, Sheem KY, Cojocaru CS, Xie SS, Lee YH (2012) Diffusion mechanism of lithium ion through basal plane of layered graphene. J Am Chem Soc 134:8646–8654
Ma C, Shao X, Cao D (2012) Nitrogen-doped graphene nanosheets as anode materials for lithium ion batteries: a first-principles study. J Mater Chem 22:8911–8915
Yu YX (2013) Can all nitrogen-doped defects improve the performance of graphene anode materials for lithium-ion batteries? Phys Chem Chem Phys 15:16819–16827
Bulusheva LG, Kanygin MA, Arkhipov VE, Popov KM, Fedoseeva YV, Smirnov DA, Okotrub AV (2017) In situ x-ray photoelectron spectroscopy study of lithium interaction with graphene and nitrogen-doped graphene films produced by chemical vapor deposition. J Phys Chem C 121:5108–5114
Inagaki M, Toyoda M, Soneda Y, Morishita T (2018) Nitrogen-doped carbon materials. Carbon 132:104–140
Zhong J, Deng J-J, Mao B-H, Xie T, Sun X-H, Mou Z-G, Hong C-H, Yang P, Wang S-D (2012) Probing solid state N-doping in graphene by X-ray absorption near-edge structure spectroscopy. Carbon 50:321–341
Bulushev DA, Zacharska M, Shlyakhova EV, Chuvilin AL, Guo Y, Beloshapkin S, Okotrub AV, Bulusheva LG (2016) Single isolated Pd2+ cations supported on N-doped carbon as active sites for hydrogen production from formic acid decomposition. ACS Catal 6(2):681–691
Bulusheva LG, Okotrub AV, Yashina LV, Velasco-Velez JJ, Usachov DY, Vyalikh DV (2018) X-ray photoelectron spectroscopy study of the interaction of lithium with graphene. Phys Sci Rev 121(9):5108–5114
Lapteva LL, Fedoseeva YV, Gevko PN, Smirnov DA, Gusel’nikov VA, Bulusheva LG, Okotrub AV (2017) X-ray spectroscopy study of lithiated graphite obtained by thermal deposition of lithium. J Struct Chem 58:1173–1179
Fedoseeva YV, Lapteva LL, Makarova AA, Bulusheva LG, Okotrub AV (2018) Charge polarization in partially lithiated single-walled carbon nanotubes. Phys Chem Chem Phys 20:22592–22599
Zhang L, Li X, Augustsson A, Lee CM, Rubensson JE, Nordgren J, Ross PN, Guo J-H (2017) Revealing the electronic structure of LiC6 by soft X-ray spectroscopy. Appl Phys Lett 110:104106. https://doi.org/10.1063/1.4978432
Larciprete R, Goldoni A, Lizzit S, Petaccia L (2005) The electronic properties of carbon nanotubes studied by high resolution photoemission spectroscopy. Appl Phys Lett 248:8–13
Shlyakhova EV, Bulusheva LG, Kanygin MA, Plyusnin PE, Kovalenko KA, Senkovskiy BV, Okotrub AV (2014) Synthesis of nitrogen-containing porous carbon using calcium oxide nanoparticles. Phys Status Solidi B 251:2607–2612
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B Condens Matter Mater Phys 37:785–789
Bochevarov AD, Harder E, Hughes TF, Greenwood JR, Braden DA, Philipp DM, Rinaldon D, Halls MD, Zhang J, Friesner RA (2013) Jaguar: a high-performance quantum chemistry software program with strengths in life and materials sciences. Int J Quantum Chem 113(18):2110–2142
Wang Q, Li H, Chen L, Huang X (2002) Novel spherical microporous carbon as anode material for Li-ion batteries. Solid State Ion 152:43–50
Tarascon J-M, Armand M (2001) Issues and challenges facing rechargeable lithium batteries. Nature 414:359–367
Billaud D, Henry F, Wellmann P (1994) Electrochemical intercalation of lithium into carbon materials. Mol Cryst Liq Cryst Sci Technol 245:159–164
Bulusheva LG, Stolyarova SG, Chuvilin AL, Shubin YV, Asanov IP, Sorokin AM, Mel’gunov MS, Zhang S, Dong Y, Chen X, Song H, Okotrub AV (2018) Creation of nanosized holes in graphene planes for improvement of rate capability of lithium-ion batteries. Nanotechnology 29:134001. https://doi.org/10.1088/1361-6528/aaa99f
Ji L, Zhang X (2009) Fabrication of porous carbon nanofibers and their application as anode materials for rechargeable lithium-ion batteries. Nanotechnology 20:155705. https://doi.org/10.1088/0957-4484/20/15/155705
Tsumura T, Arikawa A, Kinumoto T, Arai Y, Morishita T, Orikasa H, Inagaki M, Toyoda M (2014) Structure of heat-treated mesoporous carbon and its electrochemical lithium intercalation behavior. Mater Chem Phys 147:1175–1182
Jia N, Wang Z, Yang G, Shen H, Zhu L (2007) Electrochemical properties of ordered mesoporous carbon and its electroanalytical application for selective determination of dopamine. Electrochem Commun 9:233–238
Xu Z, Chen J, Zhang X, Song Q, Wu J, Ding L, Zhang C, Zhu H, Cui H (2019) Template-free preparation of nitrogen-doped activated carbon with porous architecture for high-performance supercapacitors. Microporous Mesoporous Mater 276:280–291
Chu PK, Li L (2006) Characterization of amorphous and nanocrystalline carbon films. Mater Chem Phys 96:253–277
Nikitin A, Näslund L-Å, Zhang Z, Nilsson A (2008) C–H bond formation at the graphite surface studied with core level spectroscopy. Surf Sci 602:2575–2580
Díaz J, Paolicelli G, Ferrer S, Comin F (1996) Separation of the sp 3 and sp 2 components in the C 1s photoemission spectra of amorphous carbon films. Phys Rev B 54:8064–8069
Fedoseeva YV, Pozdnyakov GA, Okotrub AV, Kanygin MA, Nastaushev YV, Vilkov OY, Bulusheva LG (2016) Effect of substrate temperature on the structure of amorphous oxygenated hydrocarbon films grown with a pulsed supersonic methane plasma flow. Appl Surf Sci 385:464–471
Yan J, Wang Q, Wei T, Jiang L, Zhang M, Jing X, Fan Z (2014) Template-assisted low temperature synthesis of functionalized graphene for ultrahigh volumetric performance supercapacitors. ACS Nano 8:4720–4729
Dai GP, Zhang JM, Deng S (2011) Synthesis and characterization of nitrogen-doped monolayer and multilayer graphene on TEM copper grids. Chem Phys Lett 516:212–215
Brühwiler PA, Maxwell AJ, Puglia C, Nilsson A, Andersson S, Mårtensson N (1995) π∗ and σ∗ excitons in C 1s absorption of graphite. Phys Rev Lett 74:614–617
Ehlert C, Unger WES, Saalfrank P (2014) C K-edge NEXAFS spectra of graphene with physical and chemical defects: a study based on density functional theory. Phys Chem Chem Phys 16:14083–14095
McCann R, Roy SS, Papakonstantinou P, Ahmad I, Maguire P, McLaughlin JA, Petaccia L, Lizzit S, Goldoni A (2005) NEXAFS study and electrical properties of nitrogen-incorporated tetrahedral amorphous carbon films. Diam Relat Mater 14:1057–1061
Okotrub AV, Yudanov NF, Tur VA, Asanov IP, Shubin YV, Vyalikh DV, Bulusheva LG (2012) Perforation of graphite in boiling mineral acid. Phys Status Solidi B 249:2620–2624
Minea TM, Bouchet-Fabre B, Lazar S, Point S, Zandbergen HW (2006) Angular and local spectroscopic analysis to probe the vertical alignment of N-doped well-separated carbon nanotubes. J Phys Chem B 110:15659–15662
Arrigo R, Schuster ME, Xie Z, Yi Y, Wowsnick G, Sun LL, Hermann KE, Friedrich M, Kast P, Hävecker M, Knop-Gericke A, Schlögl R (2015) Nature of the N–Pd Interaction in nitrogen-doped carbon nanotube catalysts. ACS Catal 5:2740–2753
Bulusheva LG, Okotrub AV, Fedoseeva YV, Kurenya AG, Asanov IP, Vilkov OY, Koósd AA, Grobert N (2015) Controlling pyridinic, pyrrolic, graphitic, and molecular nitrogen in multi-wall carbon nanotubes using precursors with different N/C ratios in aerosol assisted chemical vapor deposition. Phys Chem Chem Phys 17:23741–23747
Inagaki M, Toyod M, Soneda Y, Morishita T (2018) Nitrogen-doped carbon materials. Carbon 132:104–140
Matanovic I, Artyushkova K, Strand MB, Dzara MJ, Pylypenko S, Atanassov P (2016) Core level shifts of hydrogenated pyridinic and pyrrolic nitrogen in the nitrogen-containing graphene-based electrocatalysts: in-plane vs edge defects. J Phys Chem C120:29225–29232
Kepaptsoglou D, Hardcastle TP, Seabourne CR, Bangert U, Zan R, Amani JA, Hofsäss H, Nicholls RJ, Brydson RMD, Scott AJ, Ramasse QM (2015) Electronic structure modification of ion implanted graphene: the spectroscopic signatures of p- and n-type doping. ACS Nano 9:11398–11407
Latham KG, Dose WM, Allen JA, Donne SW (2018) Nitrogen doped heat treated and activated hydrothermal carbon: NEXAFS examination of the carbon surface at different temperatures. Carbon 128:179–190
Tenorio BNC, Oliveira RR, Nascimento MAC, Rocha AB (2018) Coupled cluster and time-dependent density functional theory description of inner shell photoabsorption cross sections of molecules. J Chem Theory Comput 14:5324–5338
Leinweber P, Kruse J, Walley FL, Gillespie A, Eckhardt K-U, Blyth R, Regier T (2007) Nitrogen K-edge XANES-an overview of reference compounds used to identify ‘unknown’ organic nitrogen in environmental samples. J Synchrotron Rad 14:500–511
Newbury DC, Ishii I, Hitchcock AP (1986) Inner shell electron-energy loss spectroscopy of some heterocyclic molecules. Can J Chem Eng 64:1145–1155
Latham KG, Simone MI, Dose WM, Allen JA, Donne SW (2017) Synchrotron based NEXAFS study on nitrogen doped hydrothermal carbon: insights into surface functionalities and formation mechanisms. Carbon 114:566–578
Acknowledgements
We thank Dr. M. A. Kanygin for the SEM images, Mr. A. V. Ishchenko for the TEM images and Dr. B. A. Kolesov for the Raman spectra. The work was financially supported by the Russian Science Foundation (Grant No. 17-73-10226). The work was partially supported by the bilateral Program “Russian-German Laboratory at BESSY II” in the part of XPS and NEXAFS measurements.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lapteva, L.L., Fedoseeva, Y.V., Shlyakhova, E.V. et al. NEXAFS spectroscopy study of lithium interaction with nitrogen incorporated in porous graphitic material. J Mater Sci 54, 11168–11178 (2019). https://doi.org/10.1007/s10853-019-03586-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-019-03586-6