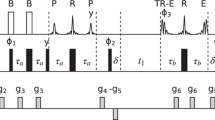
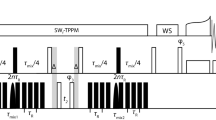
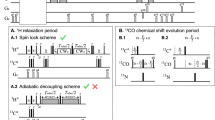
Abstract
1H–15N HSQC spectroscopy is a workhorse of protein NMR. However, under physiological conditions the quality of HSQC spectra tends to deteriorate due to fast solvent exchange. For globular proteins only a limited number of surface residues are affected, but in the case of intrinsically disordered proteins (IDPs) HSQC spectra are thoroughly degraded, suffering from both peak broadening and loss of intensity. To alleviate this problem, we make use of the following two concepts. (1) Proton-decoupled HSQC. Regular HSQC and its many variants record the evolution of multi-spin modes, 2NxHz or 2NxHx, in indirect dimension. Under the effect of fast solvent exchange these modes undergo rapid decay, which results in severe line-broadening. In contrast, proton-decoupled HSQC relies on Nx coherence which is essentially insensitive to the effects of solvent exchange. Moreover, for measurements involving IDPs at or near physiological temperature, Nx mode offers excellent relaxation properties, leading to very sharp resonances. (2) Cross-polarization 1H-to-15N transfer. If CP element is designed such as to lock both 1HN and water magnetization, the following transfer is effected: \( {\text{H}}_{\text{x}}^{\text{water}} \to {\text{H}}_{\text{x}}^{\text{N}} \to {\text{N}}_{\text{x}} . \) Thus water magnetization is successfully exploited to boost the amount of signal. In addition, CP element suffers less loss from solvent exchange, conformational exchange, and dipolar relaxation compared to the more popular INEPT element. Combining these two concepts, we have implemented the experiment termed CP-HISQC (cross-polarization assisted heteronuclear in-phase single-quantum correlation). The pulse sequence has been designed such as to preserve water magnetization and therefore can be executed with reasonably short recycling delays. In the presence of fast solvent exchange, kex ~ 100 s−1, CP-HISQC offers much better spectral resolution than conventional HSQC-type experiments. At the same time it offers up to twofold gain in sensitivity compared to plain proton-decoupled HSQC. The new sequence has been tested on the sample of drkN SH3 domain at pH 7.5, 30 °C. High-quality spectrum has been recorded in less than 1 h, containing resonances from both folded and unfolded species. High-quality spectra have also been obtained for arginine side-chain HεNε groups in the sample of short peptide Sos. For Arg side chains, we have additionally implemented (HE)NE(CD)HD experiment. Using 13C-labeled sample of Sos, we have demonstrated that proton-to-nitrogen CP transfer remains highly efficient in the presence of solvent exchange as fast as kex = 620 s−1. In contrast, INEPT transfer completely fails in this regime.








Similar content being viewed by others
Notes
The alternative sample conditions could be pH 7.2, 37 °C. These conditions are relevant for cytosol (Orij et al. 2009). They give rise to very similar kex rates and are also suitable for CP-HISQC measurements.
In principle, loss of multispin correlations due to solvent exchange can also be described as "relaxation". However, we prefer to reserve this term for faster processes—specifically for the dynamic regime where JNH/kex ≤ 1 (including Redfield limit where JNH/kex « 1 and the formula for scalar relaxation of the first kind applies (Abragam 1961)). Since in our study these conditions are not necessarily fulfilled we prefer a more general description referring to the "loss of multispin correlations due to solvent exchange" (Skrynnikov and Ernst 1999). In what follows the term "relaxation" refers to dipolar and CSA mechanisms.
This general discussion is also valid in the situation when INEPT element includes pulsed field gradients.
This parameter is the same as \( {\text {tan}} \, \uptheta \), where θ is the standard tilt angle.
With this new hardware it is likely possible to execute the CP-HISQC experiment with shorter recycling delays. If in addition one can speed up the recovery of water magnetization, e.g. by introducing paramagnetic water relaxation agents (Hiller et al. 2005; Theillet et al. 2011), this can further improve the sensitivity of the CP-HISQC experiment. One should be mindful, however, that relaxation agents may perturb a delicate conformational equilibrium in the sample of an IDP.
Note that in the case of (fully or partially) folded proteins the saturation of the water signal does not only hurt labile 1HN protons, but also those protons that are protected from solvent exchange. Specifically, the saturation is transferred via spin diffusion from solvent-exposed protons on the surface of the protein to protons in the hydrophobic core, thus reducing the amount of magnetization across the board.
Of interest, certain advanced schemes exist to align water magnetization along the effective rf field (Hansen and Kay 2007). These schemes, however, are only useful for spin-lock experiment and do not help in the case of phase-alternated pulse trains such as DIPSI-2.
References
Abragam A (1961) The principles of nuclear magnetism. Clarendon Press, Oxford
Amezcua CA, Harper SM, Rutter J, Gardner KH (2002) Structure and interactions of PAS kinase N-terminal PAS domain: model for intramolecular kinase regulation. Structure 10:1349–1361
Bai YW, Milne JS, Mayne L, Englander SW (1993) Primary structure effects on peptide group hydrogen exchange. Proteins Struct Funct Genet 17:75–86
Ban D, Gossert AD, Giller K, Becker S, Griesinger C, Lee D (2012) Exceeding the limit of dynamics studies on biomolecules using high spin-lock field strengths with a cryogenically cooled probehead. J Magn Reson 221:1–4
Bax A, Ikura M, Kay LE, Torchia DA, Tschudin R (1990) Comparison of different modes of two-dimensional reverse-correlation NMR for the study of proteins. J Magn Reson 86:304–318
Bermel W, Bertini I, Felli IC, Piccioli M, Pierattelli R (2006) 13C-detected protonless NMR spectroscopy of proteins in solution. Prog NMR Spectrosc 48:25–45
Bermel W, Bertini I, Csizmok V, Felli IC, Pierattelli R, Tompa P (2009) H-start for exclusively heteronuclear NMR spectroscopy: the case of intrinsically disordered proteins. J Magn Reson 198:275–281
Bista M, Freund SM, Fersht AR (2012) Domain-domain interactions in full-length p53 and a specific DNA complex probed by methyl NMR spectroscopy. Proc Natl Acad Sci USA 109:15752–15756
Blaum BS, Deakin JA, Johansson CM, Herbert AP, Barlow PN, Lyon M, Uhrin D (2010) Lysine and arginine side chains in glycosaminoglycan-protein complexes investigated by NMR, cross-linking, and mass spectrometry: a case study of the Factor H–Heparin interaction. J Am Chem Soc 132:6374–6381
Chevelkov V, Xue Y, Rao DK, Forman-Kay JD, Skrynnikov NR (2010) 15NH/D-SOLEXSY experiment for accurate measurement of amide solvent exchange rates. Application to denatured drkN SH3. J Biomol NMR 46:227–244
Connelly GP, Bai YW, Jeng MF, Englander SW (1993) Isotope effects in peptide group hydrogen exchange. Proteins Struct Funct Genet 17:87–92
Croke RL, Sallum CO, Watson E, Watt ED, Alexandrescu AT (2008) Hydrogen exchange of monomeric α-synuclein shows unfolded structure persists at physiological temperature and is independent of molecular crowding in Escherichia coli. Protein Sci 17:1434–1445
Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A (1995) NMRPipe: a multidimensional spectral processing system based on unix pipes. J Biomol NMR 6:277–293
Diehl C, Engstrom O, Delaine T, Hakansson M, Genheden S, Modig K, Leffler H, Ryde U, Nilsson UJ, Akke M (2010) Protein flexibility and conformational entropy in ligand design targeting the carbohydrate recognition domain of galectin-3. J Am Chem Soc 132:14577–14589
Esadze A, Li DW, Wang TZ, Bruschweiler R, Iwahara J (2011) Dynamics of lysine side-chain amino groups in a protein studied by heteronuclear 1H-15N NMR spectroscopy. J Am Chem Soc 133:909–919
Farrow NA, Zhang OW, Forman-Kay JD, Kay LE (1994) A heteronuclear correlation experiment for simultaneous determination of 15N longitudinal decay and chemical exchange rates of systems in slow equilibrium. J Biomol NMR 4:727–734
Farrow NA, Zhang OW, Forman-Kay JD, Kay LE (1995) Comparison of the backbone dynamics of a folded and an unfolded SH3 domain existing in equilibrium in aqueous buffer. Biochemistry 34:868–878
Felli IC, Brutscher B (2009) Recent advances in solution NMR: fast methods and heteronuclear direct detection. ChemPhysChem 10:1356–1368
Fernandez C, Hilty C, Wider G, Guntert P, Wuthrich K (2004) NMR structure of the integral membrane protein OmpX. J Mol Biol 336:1211–1221
Gil S, Hošek T, Solyom Z, Kümmerle R, Brutscher B, Pierattelli R, Felli IC (2013) NMR spectroscopic studies of intrinsically disordered proteins at near-physiological conditions. Angew Chem Int Edit 52:11808–11812
Gray FLV, Murai MJ, Grembecka J, Cierpicki T (2012) Detection of disordered regions in globular proteins using 13C-detected NMR. Protein Sci 21:1954–1960
Grzesiek S, Bax A (1993) The importance of not saturating H2O in protein NMR: application to sensitivity enhancement and NOE measurements. J Am Chem Soc 115:12593–12594
Haddad KC, Sudmeier JL, Bachovchin DA, Bachovchin WW (2005) α-Lytic protease can exist in two separately stable conformations with different His57 mobilities and catalytic activities. Proc Natl Acad Sci USA 102:1006–1011
Hansen DF, Kay LE (2007) Improved magnetization alignment schemes for spin-lock relaxation experiments. J Biomol NMR 37:245–255
Hiller S, Wider G, Etezady-Esfarjani T, Horst R, Wuthrich K (2005) Managing the solvent water polarization to obtain improved NMR spectra of large molecular structures. J Biomol NMR 32:61–70
Hsu STD, Bertoncini CW, Dobson CM (2009) Use of protonless NMR spectroscopy to alleviate the loss of information resulting from exchange-broadening. J Am Chem Soc 131:7222–7223
Hu F, Schmidt-Rohr K, Hong M (2012) NMR detection of pH-dependent histidine-water proton exchange reveals the conduction mechanism of a transmembrane proton channel. J Am Chem Soc 134:3703–3713
Iwahara J, Jung YS, Clore GM (2007) Heteronuclear NMR spectroscopy for lysine NH3 groups in proteins: unique effect of water exchange on 15N transverse relaxation. J Am Chem Soc 129:2971–2980
Jurt S, Zerbe O (2012) A study on the influence of fast amide exchange on the accuracy of 15N relaxation rate constants. J Biomol NMR 54:389–400
Kay LE, Keifer P, Saarinen T (1992) Pure absorption gradient enhanced heteronuclear single quantum correlation spectroscopy with improved sensitivity. J Am Chem Soc 114:10663–10665
Keifer PA (1999) 90° degrees pulse width calibrations: how to read a pulse width array. Concept Magnetic Res 11:165–180
Krishnan VV, Rance M (1995) Influence of chemical exchange among homonuclear spins in heteronuclear coherence-transfer experiments in liquids. J Magn Reson A 116:97–106
Kupce E, Freeman R (1996) Optimized adiabatic pulses for wideband spin inversion. J Magn Reson A 118:299–303
Levitt MH (1991) Heteronuclear cross polarization in liquid-state nuclear magnetic resonance: mismatch compensation and relaxation behavior. J Chem Phys 94:30–38
Loh SN, Kay MS, Baldwin RL (1995) Structure and stability of a second molten globule intermediate in the apomyoglobin folding pathway. Proc Natl Acad Sci USA 92:5446–5450
Majumdar A, Zuiderweg ERP (1995) Efficiencies of double-resonance and triple-resonance J cross-polarization in multidimensional NMR. J Magn Reson A 113:19–31
Marion D, Ikura M, Tschudin R, Bax A (1989) Rapid recording of 2D NMR spectra without phase cycling: application to the study of hydrogen exchange in proteins. J Magn Reson 85:393–399
Marsh JA, Forman-Kay JD (2009) Structure and disorder in an unfolded state under nondenaturing conditions from ensemble models consistent with a large number of experimental restraints. J Mol Biol 391:359–374
Mori S, Abeygunawardana C, Johnson MO, van Zijl PCM (1995) Improved sensitivity of HSQC spectra of exchanging protons at short interscan delays using a new fast HSQC (FHSQC) detection scheme that avoids water saturation. J Magn Reson B 108:94–98
Mueller GA, Smith AM, Chapman MD, Rule GS, Benjamin DC (2001) Hydrogen exchange nuclear magnetic resonance spectroscopy mapping of antibody epitopes on the house dust mite allergen Der p 2*. J Biol Chem 276:9359–9365
Mulder FAA, Spronk CAEM, Slijper M, Kaptein R, Boelens R (1996) Improved HSQC experiments for the observation of exchange broadened signals. J Biomol NMR 8:223–228
Muller L, Legault P, Pardi A (1995) Improved RNA structure determination by detection of NOE contacts to exchange-broadened amino protons. J Am Chem Soc 117:11043–11048
Novacek J, Zawadzka-Kazimierczuk A, Papouskova V, Zidek L, Sanderova H, Krasny L, Kozminski W, Sklenar V (2011) 5D 13C-detected experiments for backbone assignment of unstructured proteins with a very low signal dispersion. J Biomol NMR 50:1–11
Orij R, Postmus J, Ter Beek A, Brul S, Smits GJ (2009) In vivo measurement of cytosolic and mitochondrial pH using a pH-sensitive GFP derivative in Saccharomyces cerevisiae reveals a relation between intracellular pH and growth. Microbiology 155:268–278
Palmer AG, Cavanagh J, Wright PE, Rance M (1991) Sensitivty improvement in proton-detected two-dimensional heteronuclear correlation NMR spectroscopy. J Magn Reson 93:151–170
Pasat G, Zintsmaster JS, Peng JW (2008) Direct 13C detection for carbonyl relaxation studies of protein dynamics. J Magn Reson 193:226–232
Rucker SP, Shaka AJ (1989) Broad-band homonuclear cross-polarization in 2D NMR using DIPSI-2. Mol Phys 68:509–517
Schanda P, Brutscher B (2005) Very fast two-dimensional NMR spectroscopy for real-time investigation of dynamic events in proteins on the time scale of seconds. J Am Chem Soc 127:8014–8015
Schanda P, Kupce E, Brutscher B (2005) SOFAST-HMQC experiments for recording two-dimensional heteronuclear correlation spectra of proteins within a few seconds. J Biomol NMR 33:199–211
Segawa T, Kateb F, Duma L, Bodenhausen G, Pelupessy P (2008) Exchange rate constants of invisible protons in proteins determined by NMR spectroscopy. ChemBioChem 9:537–542
Shaka AJ, Keeler J, Frenkiel T, Freeman R (1983) An improved sequence for broad-band decoupling: WALTZ-16. J Magn Reson 52:335–338
Sklenar V, Piotto M, Leppik R, Saudek V (1993) Gradient-tailored water suppression for 1H–15N HSQC experiments optimized to retain full sensitivity. J Magn Reson A 102:241–245
Skrynnikov NR, Ernst RR (1999) Detection of intermolecular chemical exchange through decorrelation of two-spin order. J Magn Reson 137:276–280
Takayama Y, Castaneda CA, Chimenti M, Garcia-Moreno B, Iwahara J (2008) Direct evidence for deprotonation of a lysine side chain buried in the hydrophobic core of a protein. J Am Chem Soc 130:6714–6715
Theillet FX, Binolfi A, Liokatis S, Verzini S, Selenko P (2011) Paramagnetic relaxation enhancement to improve sensitivity of fast NMR methods: application to intrinsically disordered proteins. J Biomol NMR 51:487–495
Trbovic N, Cho JH, Abel R, Friesner RA, Rance M, Palmer AG (2009) Protein side-chain dynamics and residual conformational entropy. J Am Chem Soc 131:615–622
Vidovic V, Prongidi-Fix L, Bechinger B, Werten S (2009) Production and isotope labeling of antimicrobial peptides in Escherichia coli by means of a novel fusion partner that enables high-yield insoluble expression and fast purification. J Pept Sci 15:278–284
Werbeck ND, Kirkpatrick J, Hansen DF (2013) Probing arginine side chains and their dynamics with carbon-detected NMR spectroscopy: application to the 42 kDa human histone deacetylase 8 at high pH. Angew Chem Int Edit 52:3145–3147
Wu XD, Knudsen B, Feller SM, Zheng J, Sali A, Cowburn D, Hanafusa H, Kuriyan J (1995) Structural basis for the specific interaction of lysine-containing proline-rich peptides with the N-terminal SH3 domain of c-Crk. Structure 3:215–226
Yao SG, Hinds MG, Murphy JM, Norton RS (2011) Exchange enhanced sensitivity gain for solvent-exchangeable protons in 2D 1H–15N heteronuclear correlation spectra acquired with band-selective pulses. J Magn Reson 211:243–247
Yuwen T, Skrynnikov NR (2013) Proton-decoupled CPMG: a better experiment for measuring 15N R2 relaxation in disordered proteins. J Magn Reson [ePub ahead of print]
Zangger K, Armitage IM (1998) Sensitivity-enhanced detection of fast exchanging protons by an exchange-edited gradient HEHAHA-HSQC experiment. J Magn Reson 135:70–75
Zhang O, Forman-Kay JD (1995) Structural characterization of a folded and an unfolded state of an SH3 domain in aqueous buffer and solution structure of this domain by NMR. Biochemistry 34:6784–6794
Zhang OW, Kay LE, Olivier JP, Forman-Kay JD (1994) Backbone 1H and 15N resonance assignments of the N-terminal SH3 domain of drk in folded and unfolded states using enhanced-sensitivity pulsed-field gradient NMR techniques. J Biomol NMR 4:845–858
Acknowledgments
This study was supported by the funds from NSF Grant MCB 1158347.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yuwen, T., Skrynnikov, N.R. CP-HISQC: a better version of HSQC experiment for intrinsically disordered proteins under physiological conditions. J Biomol NMR 58, 175–192 (2014). https://doi.org/10.1007/s10858-014-9815-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10858-014-9815-5