Abstract
The 2019 ISMAR Prize recognized NMR studies of disordered proteins. Here we provide a highly personal perspective on the discovery of intrinsically disordered proteins and the development and application of NMR methods to characterize their conformational ensembles, dynamics, and interactions.

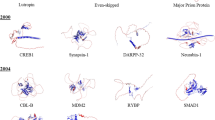
Figure adapted from reference (Dyson and Wright 2005) with permission


Adapted from reference (Berlow et al. 2017), with permission

Reproduced from reference (Berlow et al. 2017), with permission
Similar content being viewed by others
References
Arai M, Ferreon JC, Wright PE (2012) Quantitative analysis of multisite protein-ligand interactions by NMR: binding of intrinsically disordered p53 transactivation subdomains with the TAZ2 domain of CBP. J Am Chem Soc 134:3792–3803
Arai M, Sugase K, Dyson HJ, Wright PE (2015) Conformational propensities of intrinsically disordered proteins influence the mechanism of binding and folding. Proc Natl Acad Sci USA 112:9614–9619
Bae SH, Dyson HJ, Wright PE (2009) Prediction of the rotational tumbling time for proteins with disordered segments. J Am Chem Soc 131:6814–6821
Berlow RB, Dyson HJ, Wright PE (2017) Hypersensitive termination of the hypoxic response by a disordered protein switch. Nature 543:447–451
Berlow RB, Martinez-Yamout MA, Dyson HJ, Wright PE (2019) role of backbone dynamics in modulating the interactions of disordered ligands with the TAZ1 domain of the CREB-binding protein. Biochemistry 58:1354–1362
Borg M, Mittag T, Pawson T, Tyers M, Forman-Kay JD, Chan HS (2007) Polyelectrostatic interactions of disordered ligands suggest a physical basis for ultrasensitivity. Proc Natl Acad Sci USA 104:9650–9655
Borgia A, Borgia MB, Bugge K, Kissling VM, Heidarsson PO, Fernandes CB, Sottini A, Soranno A, Buholzer KJ, Nettels D, Kragelund BB, Best RB, Schuler B (2018) Extreme disorder in an ultrahigh-affinity protein complex. Nature 555:61–66
Brown JE, Klee WA (1971) Helix-coil transition of the isolated amino terminus of ribonuclease. Biochemistry 10:470–476
Dames SA, Martinez-Yamout M, De Guzman RN, Dyson HJ, Wright PE (2002) Structural basis for Hif-1α/CBP recognition in the cellular hypoxic response. Proc Natl Acad Sci USA 99:5271–5276
Daniels A, Williams RJP, Wright PE (1976) Nuclear magnetic resonance studies of the adrenal gland and some other organs. Nature 261:321–323
Daniels AJ, Williams RJP, Wright PE (1978) The character of the stored molecules in chromaffin granules of the adrenal medulla: a nuclear magnetic resonance study. Neuroscience 3:573–585
De Guzman RN, Martinez-Yamout M, Dyson HJ, Wright PE (2004) Interaction of the TAZ1 domain of CREB-binding protein with the activation domain of CITED2: regulation by competition between intrinsically unstructured ligands for non-identical binding sites. J Biol Chem 279:3042–3049
Demarest SJ, Martinez-Yamout M, Chung J, Chen H, Xu W, Dyson HJ, Evans RM, Wright PE (2002) Mutual synergistic folding in recruitment of CBP/p300 by p160 nuclear receptor coactivators. Nature 415:549–553
Dunker AK, Lawson JD, Brown CJ, Williams RM, Romero P, Oh JS, Oldfield CJ, Campen AM, Ratliff CM, Hipps KW, Ausio J, Nissen MS, Reeves R, Kang C, Kissinger CR, Bailey RW, Griswold MD, Chiu W, Garner EC, Obradovic Z (2001) Intrinsically disordered protein. J Mol Graph Model 19:26–59
Dunker AK, Babu MM, Barbar E, Blackledge M, Bondos SE, Dosztanyi Z, Dyson HJ, Forman-Kay J, Fuxreiter M, Gsponer J, Han KH, Jones DT, Longhi S, Metallo SJ, Nishikawa K, Nussinov R, Obradovic Z, Pappu RV, Rost B, Selenko P, Subramaniam V, Sussman JL, Tompa P, Uversky VN (2013) What’s in a name? Why these proteins are intrinsically disordered. Intrins Disord Prot 1:e24157
Dyson HJ, Wright PE (1991) Defining solution conformations of small linear peptides. Annu Rev Biophys Biophys Chem 20:519–538
Dyson HJ, Wright PE (2005) Intrinsically unstructured proteins and their functions. Nat Rev Mol Cell Biol 6:197–208
Dyson HJ, Wright PE (2017) How does your protein fold? Elucidating the apomyoglobin folding pathway. Acc Chem Res 50:105–111
Dyson HJ, Cross KJ, Houghten RA, Wilson IA, Wright PE, Lerner RA (1985) The immunodominant site of a synthetic immunogen has a conformational preference in water for a Type-II reverse turn. Nature 318:480–483
Dyson HJ, Rance M, Houghten RA, Lerner RA, Wright PE (1988a) Folding of immunogenic peptide fragments of proteins in water solution. I. Sequence requirements for the formation of a reverse turn. J Mol Biol 201:161–200
Dyson HJ, Rance M, Houghten RA, Wright PE, Lerner RA (1988b) Folding of immunogenic peptide fragments of proteins in water solution. II. The nascent helix. J Mol Biol 201:201–217
Dyson HJ, Gippert GP, Case DA, Holmgren A, Wright PE (1990) Three-dimensional solution structure of the reduced form of Escherichia coli thioredoxin determined by nuclear magnetic resonance spectroscopy. Biochemistry 29:4129–4136
Dyson HJ, Merutka G, Waltho JP, Lerner RA, Wright PE (1992a) Folding of peptide fragments comprising the complete sequence of proteins. Models for initiation of protein folding. I. Myohemerythrin. J Mol Biol 226:795–817
Dyson HJ, Sayre JR, Merutka G, Shin H-C, Lerner RA, Wright PE (1992b) Folding of peptide fragments comprising the complete sequence of proteins: models for the initiation of protein folding. II. Plastocyanin. J Mol Biol 226:819–835
Eliezer D, Yao J, Dyson HJ, Wright PE (1998) Structural and dynamic characterization of partially folded states of apomyoglobin and implications for protein folding. Nat Struct Biol 5:148–155
Elkins JM, Hewitson KS, McNeill LA, Seibel JF, Schlemminger I, Pugh CW, Ratcliffe PJ, Schofield CJ (2003) Structure of factor-inhibiting hypoxia-inducible factor (HIF) reveals mechanism of oxidative modification of HIF-1α. J Biol Chem 278(3):1802–1806
Freedman SJ, Sun ZY, Poy F, Kung AL, Livingston DM, Wagner G, Eck MJ (2002) Structural basis for recruitment of CBP/p300 by hypoxia-inducible factor-1a. Proc Natl Acad Sci USA 99:5367–5372
Gibbs EB, Cook EC, Showalter SA (2017) Application of NMR to studies of intrinsically disordered proteins. Arch Biochem Biophys 628:57–70
Gillespie JR, Shortle D (1997) Characterization of long-range structure in the denatured state of staphylococcal nuclease. I. Paramagnetic relaxation enhancement by nitroxide spin labels. J Mol Biol 268:158–169
Gronenborn AM, Bax A, Wingfield PT, Clore GM (1989) A powerful method of sequential proton resonance assignment in proteins using relayed 15N-1H multiple quantum coherence spectroscopy. FEBS Lett 243:93–98
Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG (2001) HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292(5516):464–468
Jaakkola P, Mole DR, Tian Y-M, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ (2001) Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292(5516):468–472
Janin J, Sternberg MJ (2013) Protein flexibility, not disorder, is intrinsic to molecular recognition. F1000 Biol Rep 5:2
Jeener J, Meier BH, Bachman P, Ernst RR (1979) Investigation of exchange processes by two-dimensional NMR spectroscopy. J Chem Phys 71:4546–4553
Jensen MR, Zweckstetter M, Huang J-R, Blackledge M (2014) Exploring free-energy landscapes of intrinsically disordered proteins at atomic resolution using nmr spectroscopy. Chem Rev 114:6632–6660
Konrat R (2014) NMR contributions to structural dynamics studies of intrinsically disordered proteins. J Magn Reson 241:74–85
Kriwacki RW, Hengst L, Tennant L, Reed SI, Wright PE (1996) Structural studies of p21Waf1/Cip1/Sdi1 in the free and Cdk2-bound state: conformational disorder mediates binding diversity. Proc Natl Acad Sci USA 93:11504–11509
Krois AS, Ferreon JC, Martinez-Yamout MA, Dyson HJ, Wright PE (2016) Recognition of the disordered p53 transactivation domain by the transcriptional adapter zinc finger domains of CREB-binding protein. Proc Natl Acad Sci USA 113:E1853–E1862
Krois AS, Dyson HJ, Wright PE (2018) Long-range regulation of p53 DNA binding by its intrinsically disordered N-terminal transactivation domain. Proc Natl Acad Sci USA 115:E11302–E11310
Land D, Peet DJ, Whelan DA, Gorman JJ, Whitelaw ML (2002) Asparagine hydroxylation on the HIF transactivation domain: a hypoxic switch. Science 295:858–861
Lee MS, Gippert G, Soman KY, Case DA, Wright PE (1989) Three-dimensional solution structure of a single zinc finger binding domain. Science 245:635–637
Lee CW, Arai M, Martinez-Yamout MA, Dyson HJ, Wright PE (2009) Mapping the interactions of the p53 transactivation domain with the KIX domain of CBP. Biochemistry 48:2115–2124
Lietzow MA, Jamin M, Dyson HJ, Wright PE (2002) Mapping long-range contacts in a highly unfolded protein. J Mol Biol 322:655–662
Love JJ, Li XA, Case DA, Giese K, Grosschedl R, Wright PE (1995) Structural basis for DNA bending by the architectural transcription factor LEF-1. Nature 376:791–795
Love JJ, Li X, Chung J, Dyson HJ, Wright PE (2004) The LEF-1 HMG domain undergoes a disorder-to-order transition upon complex formation with cognate DNA. Biochemistry 43:8725–8734
Marsh JA, Forman-Kay JD (2012) Ensemble modeling of protein disordered states: experimental restraint contributions and validation. Prot Struct Func Bioinform 80:556–572
Milles S, Salvi N, Blackledge M, Jensen MR (2018) Characterization of intrinsically disordered proteins and their dynamic complexes: from in vitro to cell-like environments. Prog NMR Spect 109:79–100
Mohana-Borges R, Goto NK, Kroon GJA, Dyson HJ, Wright PE (2004) Structural characterization of unfolded states of apomyoglobin using residual dipolar couplings. J Mol Biol 340:1131–1142
Moore JM, Case DA, Chazin WJ, Gippert GP, Havel TF, Powls R, Wright PE (1988) Three-dimensional solution structure of plastocyanin from the green alga Scenedesmus obliquus. Science 240:314–317
Neri D, Billeter M, Wider G, Wüthrich K (1992) NMR determination of residual structure in a urea-denatured protein, the 434-repressor. Science 257:1559–1563
Nielsen JT, Mulder FAA (2018) POTENCI: prediction of temperature, neighbor and pH-corrected chemical shifts for intrinsically disordered proteins. J Biomol NMR 70:141–165
Niman HL, Houghten RA, Walker LE, Reisfeld RA, Wilson IA, Hogle JM, Lerner RA (1983) Generation of protein-reactive antibodies by short peptides is an event of high frequency: implications for the structural basis of immune recognition. Proc Natl Acad Sci USA 80:4949–4953
Radhakrishnan I, Pérez-Alvarado GC, Parker D, Dyson HJ, Montminy MR, Wright PE (1997) Solution structure of the KIX domain of CBP bound to the transactivation domain of CREB: a model for activator:coactivator interactions. Cell 91:741–752
Radhakrishnan I, Pérez-Alvarado GC, Dyson HJ, Wright PE (1998) Conformational preferences in the Ser133-phosphorylated and non-phosphorylated forms of the kinase inducible transactivation domain of CREB. FEBS Lett 430:317–322
Reymond MT, Merutka G, Dyson HJ, Wright PE (1997) Folding propensities of peptide fragments of myoglobin. Protein Sci 6:706–716
Romero P, Obradovic Z, Kissinger CR, Villafranca JE, Dunker AK (1997) Identifying disordered regions in proteins from amino acid sequences. In: Proceedings of IEEE International Conference on Neural Networks pp 90–95
Schmid T, Zhou J, Brune B (2004) HIF-1 and p53: communication of transcription factors under hypoxia. J Cell Mol Med 8:423–431
Schwarzinger S, Kroon GJA, Foss TR, Wright PE, Dyson HJ (2000) Random coil chemical shifts in acidic 8 M urea: implementation of random coil chemical shift data in NMRView. J Biomol NMR 18:43–48
Schwarzinger S, Kroon GJA, Foss TR, Chung J, Wright PE, Dyson HJ (2001) Sequence dependent correction of random coil NMR chemical shifts. J Am Chem Soc 123:2970–2978
Schwarzinger S, Wright PE, Dyson HJ (2002) Molecular hinges in protein folding: the urea-denatured state of apomyoglobin. Biochemistry 41:12681–12686
Sciolino N, Burz DS, Shekhtman A (2019) In-cell NMR spectroscopy of intrinsically disordered proteins. Proteomics 19:1800055
Semenza GL (2004) Hydroxylation of HIF-1: oxygen sensing at the molecular level. Physiology 19:176–182
Sugase K, Dyson HJ, Wright PE (2007) Mechanism of coupled folding and binding of an intrinsically disordered protein. Nature 447:1021–1025
Takeuchi K, Arthanari H, Shimada I, Wagner G (2015) Nitrogen detected TROSY at high field yields high resolution and sensitivity for protein NMR. J Biomol NMR 63:323–331
Tamiola K, Acar B, Mulder FAA (2010) Sequence-specific random coil chemical shifts of intrinsically disordered proteins. J Am Chem Soc 132:18000–18003
Theillet FX, Binolfi A, Frembgen-Kesner T, Hingorani K, Sarkar M, Kyne C, Li C, Crowley PB, Gierasch L, Pielak GJ, Elcock AH, Gershenson A, Selenko P (2014) Physicochemical properties of cells and their effects on intrinsically disordered proteins (IDPs). Chem Rev 114:6661–6714
Theillet F-X, Binolfi A, Bekei B, Martorana A, Rose HM, Stuiver M, Verzini S, Lorenz D, van Rossum M, Goldfarb D, Selenko P (2016) Structural disorder of monomeric α-synuclein persists in mammalian cells. Nature 530:45–50
Tompa P, Fuxreiter M (2008) Fuzzy complexes: polymorphism and structural disorder in protein-protein interactions. Trends Biochem Sci 33:2–8
Tsvetkov P, Reuven N, Prives C, Shaul Y (2009) Susceptibility of p53 unstructured N terminus to 20 S proteasomal degradation programs the stress response. J Biol Chem 284:26234–26242
van der Lee R, Buljan M, Lang B, Weatheritt RJ, Daughdrill GW, Dunker AK, Fuxreiter M, Gough J, Gsponer J, Jones DT, Kim PM, Kriwacki RW, Oldfield CJ, Pappu RV, Tompa P, Uversky VN, Wright PE, Babu MM (2014a) Classification of intrinsically disordered regions and proteins. Chem Rev 114:6589–6631
van der Lee R, Lang B, Kruse K, Gsponer J, Sánchez de Groot N, Huynen Martijn A, Matouschek A, Fuxreiter M, Babu MM (2014b) Intrinsically disordered segments affect protein half-life in the cell and during evolution. Cell Rep 8:1832–1844
Viles JH, Donne DG, Kroon GJA, Prusiner SB, Cohen FE, Dyson HJ, Wright PE (2001) Local structural plasticity of the prion protein. Analysis of NMR relaxation dynamics. Biochemistry 40:2743–2753
Wilson IA, Wiley DC, Skehel JJ (1981) Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A. Nature 289:366–373
Wokaun A, Ernst RR (1977) Selective detection of multiple quantum transitions in NMR by two-dimensional spectroscopy. Chem Phys Lett 52:407–412
Wright PE, Dyson HJ (1999) Intrinsically unstructured proteins: re-assessing the protein structure-function paradigm. J Mol Biol 293:321–331
Wright PE, Dyson HJ (2015) Intrinsically disordered proteins in cellular signalling and regulation. Nat Rev Mol Cell Biol 16:18–29
Wright PE, Dyson HJ, Lerner RA (1988) Conformation of peptide fragments of proteins in aqueous solution: implications for initiation of protein folding. Biochemistry 27:7167–7175
Yao J, Dyson HJ, Wright PE (1997) Chemical shift dispersion and secondary structure prediction in unfolded and partly folded proteins. FEBS Lett 419:285–289
Yao J, Chung J, Eliezer D, Wright PE, Dyson HJ (2001) NMR structural and dynamic characterization of the acid-unfolded state of apomyoglobin provides insights into the early events in protein folding. Biochemistry 40:3561–3571
Acknowledgements
We thank the many members of the Wright and Dyson laboratories, past and present, who have made essential contributions to the IDP work. Sadly, only a few examples of their contributions could be highlighted here. Our work on disordered proteins has been supported by the National Institutes of Health for over 30 years, through grants AI19499 and CA27489 (on anti-peptide antibodies), GM38794 (on peptides), NS14069 and AG21601 (on prions), DK34909 (on protein folding), GM57374 (on peptide NMR), CA96865 (on CBP), CA229652 (on HIF-1α/CITED2 competition), GM113251 (on chaperone interactions), GM56879 and GM075995 (on protein dynamics and p53), and CA214054 (on CREB). We also thank the Skaggs Institute for Chemical Biology for long-term support of our work.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dyson, H.J., Wright, P.E. Perspective: the essential role of NMR in the discovery and characterization of intrinsically disordered proteins. J Biomol NMR 73, 651–659 (2019). https://doi.org/10.1007/s10858-019-00280-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10858-019-00280-2