Abstract
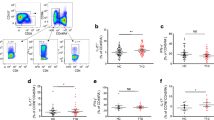
Type 1 diabetes is an autoimmune process predominantly T-cell mediated. CD26 plays a role in T-cell costimulation, migration, memory development, thymic maturation and emigration patterns. In peripheral blood from 55 patients with type 1 diabetes and 20 healthy controls, CD4+ and CD8+ T cells expressing CD26 were differentiated into naïve (N, CD45RA+CCR7+), central memory (CM, CD45RA−CCR7+), effector memory (EM, CD45RA−CCR7−), and terminally differentiated effector memory (TEMRA, CD45RA+CCR7−). In type 1 diabetes, CD4+ and CD8+ T cells expressing CD26 showed a distinctive differentiation profile: percentages and absolute numbers of CM and N cells were reduced, whereas those of TEMRA cells were markedly increased. The indices of intermediate- and long-term glycaemic control were associated negatively with the number of CM and N cells while positively with the number of TEMRA cells. The considerable accumulation of TEMRA T cells in our patients suggests life-long stimulation by protracted antigen exposure (viruses, other agents or residual self-antigens?) or a homeostatic defect in the regulation/contraction of immune responses.




Similar content being viewed by others

References
Ohnuma K, Dang NH, Morimoto C. Revisiting an old acquaintance: CD26 and its molecular mechanisms in T cell function. Trends Immunol. 2008;29:295–301.
Matteucci E, Giampietro O. Dipeptidyl peptidase-4 (CD26): knowing the function before inhibiting the enzyme. Curr Med Chem. 2009;16:2943–51.
Martinez-Navio JM, Casanova V, Pacheco R, Naval-Macabuhay I, Climent N, Garcia F, et al. Adenosine deaminase potentiates the generation of effector, memory, and regulatory CD4+ T cells. J Leukoc Biol. 2011;89:127–36.
Liu Z, Christensson M, Forslöw A, De Meester I, Sundqvist KG. A CD26-controlled cell surface cascade for regulation of T cell motility and chemokine signals. J Immunol. 2009;183:3616–24.
Klemann C, Schade J, Pabst R, Leitner S, Stiller J, von Hörsten S, et al. CD26/dipeptidyl peptidase 4-deficiency alters thymic emigration patterns and leukocyte subsets in F344-rats age-dependently. Clin Exp Immunol. 2009;155:357–65.
Tian L, Gao J, Hao J, Zhang Y, Yi H, O’Brien TD, et al. Reversal of new-onset diabetes through modulating inflammation and stimulating beta-cell replication in nonobese diabetic mice by a dipeptidyl peptidase IV inhibitor. Endocrinology. 2010;151:3049–60.
Kim SJ, Nian C, Doudet DJ, McIntosh CH. Dipeptidyl peptidase IV inhibition with MK0431 improves islet graft survival in diabetic NOD mice partially via T-cell modulation. Diabetes. 2009;58:641–51.
Ibegbu CC, Xu YX, Fillos D, Radziewicz H, Grakoui A, Kourtis AP. Differential expression of CD26 on virus-specific CD8(+) T cells during active, latent and resolved infection. Immunology. 2009;126:346–53.
Matteucci E, Ghimenti M, Consani C, Di Beo S, Giampietro O. About CD26 CD8 lymphocytes in type 1 diabetes mellitus. Scand J Immunol. 2010;71:123–4.
Monti P, Heninger AK, Bonifacio E. Differentiation, expansion, and homeostasis of autoreactive T cells in type 1 diabetes mellitus. Curr Diab Rep. 2009;9:113–8.
Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–63.
Pearce EL. Metabolism of T cell activation and differentiation. Curr Opin Immunol. 2010;22:314–20.
Viglietta V, Kent SC, Orban T, Hafler DA. GAD65-reactive T cells are activated in patients with autoimmune type 1a diabetes. J Clin Invest. 2002;109:895–903.
Hofer J, Hofer S, Zlamy M, Jeller V, Koppelstaetter C, Brandstätter A, et al. Elevated proportions of recent thymic emigrants in children and adolescents with type 1 diabetes. Rejuvenation Res. 2009;12:311–20.
Mikulkova Z, Praksova P, Stourac P, Bednarik J, Strajtova L, Pacasova R, et al. Numerical defects in CD8+CD28− T-suppressor lymphocyte population in patients with type 1 diabetes mellitus and multiple sclerosis. Cell Immunol. 2010;262:75–9.
Hedman M, Faresjö M, Axelsson S, Ludvigsson J, Casas R. Impaired CD4 and CD8 T cell phenotype and reduced chemokine secretion in recent-onset type 1 diabetic children. Clin Exp Immunol. 2008;153:360–8.
Bell EB, Westermann J. CD4 memory T cells on trial: immunological memory without a memory T cell. Trends Immunol. 2008;29:405–11.
Macallan DC, Wallace D, Zhang Y, De Lara C, Worth AT, Ghattas H, et al. Rapid turnover of effector-memory CD4(+) T cells in healthy humans. J Exp Med. 2004;200:255–60.
Gerlach C, van Heijst JW, Schumacher TN. The descent of memory T cells. Ann N Y Acad Sci. 2011;1217:139–53.
Geginat J, Lanzavecchia A, Sallusto F. Proliferation and differentiation potential of human CD8+ memory T-cell subsets in response to antigen or homeostatic cytokines. Blood. 2003;101:4260–6.
Harari A, Enders FB, Cellerai C, Bart PA, Pantaleo G. Distinct profiles of cytotoxic granules in memory CD8 T cells correlate with function, differentiation stage, and antigen exposure. J Virol. 2009;83:2862–71.
Dunne PJ, Belaramani L, Fletcher JM, Fernandez de Mattos S, Lawrenz M, Soares MV, et al. Quiescence and functional reprogramming of Epstein–Barr virus (EBV)-specific CD8+ T cells during persistent infection. Blood. 2005;106:558–65.
Koch S, Larbi A, Derhovanessian E, Ozcelik D, Naumova E, Pawelec G. Multiparameter flow cytometric analysis of CD4 and CD8 T cell subsets in young and old people. Immun Ageing. 2008;5:6.
Harari A, Vallelian F, Pantaleo G. Phenotypic heterogeneity of antigen-specific CD4 T cells under different conditions of antigen persistence and antigen load. Eur J Immunol. 2004;34:3525–33.
Appay V, van Lier RA, Sallusto F, Roederer M. Phenotype and function of human T lymphocyte subsets: consensus and issues. Cytometry A. 2008;73:975–83.
Ilonen J, Surcel HM, Käär ML. Abnormalities within CD4 and CD8 T lymphocytes subsets in type 1 (insulin-dependent) diabetes. Clin Exp Immunol. 1991;85:278–81.
Smerdon RA, Peakman M, Hussain MJ, Alviggi L, Watkins PJ, Leslie RD, et al. Increase in simultaneous coexpression of naive and memory lymphocyte markers at diagnosis of IDDM. Diabetes. 1993;42:127–33.
Rowe PA, Campbell-Thompson ML, Schatz DA, Atkinson MA. The pancreas in human type 1 diabetes. Semin Immunopathol. 2011;33:29–43.
Ramakrishnan P, Kahn DA, Baltimore D. Anti-apoptotic effect of hyperglycemia can allow survival of potentially autoreactive T cells. Cell Death Differ. 2010;18:690–9. doi:10.1038/cdd.2010.163.
Kowluru RA, Chan PS. Metabolic memory in diabetes—from in vitro oddity to in vivo problem: role of apoptosis. Brain Res Bull. 2010;81:297–302.
Gu N, Tsuda M, Matsunaga T, Adachi T, Yasuda K, Ishihara A, et al. Glucose regulation of dipeptidyl peptidase IV gene expression is mediated by hepatocyte nuclear factor-1alpha in epithelial intestinal cells. Clin Exp Pharmacol Physiol. 2008;35:1433–9.
Goldberg RB. Cytokine and cytokine-like inflammation markers, endothelial dysfunction, and imbalanced coagulation in development of diabetes and its complications. J Clin Endocrinol Metab. 2009;94:3171–82.
Acknowledgement
The authors wish to thank Dr. C. Consani for her technical assistance.
Competing Interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matteucci, E., Ghimenti, M., Di Beo, S. et al. Altered Proportions of Naïve, Central Memory and Terminally Differentiated Central Memory Subsets among CD4+ and CD8+ T Cells Expressing CD26 in Patients with Type 1 Diabetes. J Clin Immunol 31, 977–984 (2011). https://doi.org/10.1007/s10875-011-9573-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-011-9573-z