Abstract
Mosquito-borne diseases represent a major human and animal health problem in all tropical and subtropical countries worldwide. In this study, we investigated the one-pot synthesis of silver nanoparticles (AgNPs) using a cheap leaf extract of Carissa carandas (Apocynaceae). Bio-reduced AgNPs were characterized by UV–visible spectrophotometry, Fourier transform infrared spectroscopy, X-ray diffraction analysis, atomic force microscopy, scanning electron microscopy and transmission electron microscopy. The acute toxicity of C. carandas extract and green-synthesized AgNPs was evaluated on eggs and larvae of Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus. AgNPs showed high toxicity against A. stephensi, A. aegypti, and C. quinquefasciatus larvae with LC50 values of 14.33, 15.69 and 16.95 μg/mL, respectively. A single treatment with AgNPs tested at 60 μg/mL led to no egg hatchability. The egg rafts of C. quinquefasciatus were more resistant to the toxic action of AgNPs if compared to A. aegypti and A. stephensi. C. carandas-fabricated AgNPs were found safer to non-target organisms Anisops bouvieri, Diplonychus indicus and Gambusia affinis, with LC50 ranging from 1097.87 to 17249.89 µg/ml. Overall, this research shed light on the mosquitocidal potential of C. carandas, a potential bio-resource for rapid, cheap and effective synthesis of poly-disperse and stable silver nanocrystals.
Similar content being viewed by others
Introduction
Mosquitoes (Diptera: Culicidae) are vectors of many diseases, including malaria, filariasis, dengue, West Nile virus, yellow fever, chikungunya, Zika virus and Japanese encephalitis. Among them, malaria, spread by Anopheles females, dengue, spread by Aedes mosquito and filariasis, spread by Culex mosquito, are the three vector borne diseases characterizing tropical and subtropical regions worldwide, considered as major public health concerns [1]. The better strategy to lower the incidence of mosquito-transmitted diseases is to avoid mosquito biting using skin repellents, insecticide treated bed nets, and targeting mosquito young instars with pesticides [2]. Mosquito young instars have less mobility in breeding habitat so devising control measures at this stage are comparatively easy [3]. Current practice aimed to the control of mosquito larvae mainly rely to the use of synthetic pesticides such as carbamates, organophosphates and pyrethroids, as well as microbial control agents. In the early days of their use, insecticides showed success in reducing vector populations but the frequent and blind use of them increased the selection pressure on mosquitoes creating resistance and also had negative impact on human health and non-target species [4, 5]. In view of these facts, plant-borne insect control tools can be considered a safer and effective alternative [6–9].
Nanotechnology is a growing field making an outstanding impact in all spheres of human life [10]. Nanomaterials can be synthesized by different methods including chemical, physical, irradiation, and biological methods. The development of new chemical or physical methods has resulted in environmental contaminations, since the chemical procedures involved in the synthesis of nanomaterials generate a large amount of hazardous byproducts [11]. Thus, there is a need for “green nanotechnology” that includes a clean, safe, eco-friendly, and environmentally nontoxic method of nanoparticle synthesis, and in this method there is no need to use high pressure, energy, temperature, and toxic chemicals [12, 13]. The biological methods include synthesis of nanomaterials from the extracts of plant, bacterial and fungal species [14].
Currently, a growing number of plants have been recently screened for nanosynthesis of silver nanomosquitocides, including Sida acuta [15], Barleria cristata [16], Gmelina asiatica [10], Chomelia asiatica [17], Feronia elephantum [18], Heliotropium indicum [19], Clerodendrum chinense [20], Bauhinia variegata [21] and Anisomeles indica [22]. Phyto-synthesized silver nanoparticles (AgNPs) have been recently proposed as effective mosquito larvicides, and are gaining importance over synthetic chemical pesticides because of their reduced harmful effects to non-targeted species and novelty in the mechanism(s) of action [23, 24].
Carissa carandas Linn. is a large dichotomously branched evergreen shrub with short stem and strong thorns in pairs. It belongs to the family of Apocynaceae, and is commonly known as Christ’s thorn or Bengal Currant, ‘Kalakke’ in Tamil [25]. The plant is native and common throughout India, Sri Lanka, Java, Malaysia, Myanmar and Pakistan. In traditional and folk medicine, this species is widely used as an anthelmintic, astringent, appetizer, antipyretic, in biliary, stomach disorders, rheumatism and disease of the brain [26]. Earlier studies have shown that the extract of the plant possesses cardiotonic, antipyretic and antiviral activity [27, 28]. Various cardiac glycosides, a triterpenoidal constituent carissone and β-sitosterol were reported from the root extract of the plant [29]. However, to the best of our knowledge, its mosquitocidal activity is currently unknown.
In this research, we proposed a cheap and rapid method of one-pot bio-fabrication of poly-dispersed AgNPs using the aqueous leaf extract of C. carandas. Bio-reduced AgNPs were characterized by UV–vis spectrophotometry, Fourier transform infrared spectroscopy (FTIR), X-ray diffraction analysis (XRD), atomic force microscopy (AFM), scanning electron microscopy (SEM) and transmission electron microscopy (TEM). The acute toxicity of C. carandas leaf extract and green-synthesized AgNPs was evaluated against the eggs and larvae of malaria vector A. stephensi, the dengue vector A. aegypti and the filariasis vector C. quinquefasciatus. Furthermore, we evaluated the biotoxicity of C. carandas aqueous extract and green-synthesized AgNPs on three non-target aquatic mosquito predators sharing the same ecological niche of Anopheles and Aedes mosquitoes, Anisops bouvieri, Diplonychus indicus and Gambusia affinis.
Materials and Methods
Chemicals and Plant Materials
Silver nitrate was purchased by Merck (India). The glassware was acid-washed thoroughly and then rinsed with Millipore Milli-Q water. Healthy and fresh leaves of C. carandas were collected from Nilgiris, Western Ghats (11°10′N to 11°45′N latitude and 76°14′E to 77°2′E longitude), Tamil Nadu State, India. The identity was confirmed at the Department of Botany, Annamalai University, Annamalai Nagar, Tamil Nadu. Voucher specimens were numbered and kept in our laboratory and are available upon request.
Preparation of plant extracts
Leaves of C. carandas were dried in the shade and ground to fine powder in an electric grinder. Aqueous extract was prepared by mixing 50 g of dried leaf powder with 500 mL of water (boiled and cooled distilled water) with constant stirring on a magnetic stirrer. The suspension of dried leaf powder in water was left for 3 h and filtered through Whatman n. 1 filter paper and the aqueous filtrate were stored in an amber-colored airtight bottle at 10 °C temperature until testing.
Green Synthesis and Characterization of Silver Nanoparticles
Ten grams of thoroughly washed and finely cut leaves were added in a 300-mL Erlenmeyer flask along with 100 mL of sterilized double-distilled water, the mixture was boiled for 5 min before finally decanting it. The colloidal extract was filtered with Whatman filter paper n. 1, stored at −15 °C and tested within a week. The filtrate was treated with aqueous 1 mM AgNO3 (21.2 mg of AgNO3 powder in 125 mL of Milli-Q water) solution in an Erlenmeyer flask and incubated at room temperature. Eighty-eight milliliters of an aqueous solution of 1 mM silver nitrate was reduced using 12 mL of leaf extract at room temperature for 10 min, resulting in a brown–yellow solution indicating the formation of AgNPs.
The bioreduction of Ag+ ions was monitored using a UV–vis spectrophotometer (UV-160v, Shimadzu, Japan). Analysis on size, morphology, agglomeration pattern and dispersed nature of AgNPs were performed by atomic force microscopy (Agilent Technologies AFM- 5500), scanning electron microscopy (Hitachi S3000 H SEM) and transmission electron microscopy (TEM Technite 10 Philips). The purified AgNPs were examined for the presence of biomolecules using FTIR spectroscopy (Thermo Scientific Nicolet 380 FT-IR Spectrometer) KBr pellets and crystalline AgNPs were determined by XRD analysis.
Mosquito Rearing
Following the method by Govindarajan and Sivakumar [30], laboratory-bred pathogen-free strains of mosquitoes were reared in the vector control laboratory, Department of Zoology, Annamalai University. At the time of adult feeding, these mosquitoes were 3–4 days old after emergences (maintained on raisins and water) and were starved for 12 h before feeding. Each time, 500 mosquitoes per cage were fed on blood using a feeding unit fitted with Parafilm as membrane for 4 h. A. aegypti feeding was done from 12 noon to 4.00 p.m. and A. stephensi and C. quinquefasciatus were fed during 6.00 to 10.00 p.m. A membrane feeder with the bottom end fitted with Parafilm was placed with 2.0 ml of the blood sample (obtained from a slaughterhouse by collecting in a heparinized vial and stored at 4 °C) and kept over a netted cage of mosquitoes. The blood was stirred continuously using an automated stirring device, and a constant temperature of 37 °C were maintained using a water jacket circulating system. After feeding, the fully engorged females were separated and maintained on raisins. Mosquitoes were held at 28 ± 2 °C, 70–85 % relative humidity, with a photoperiod of 12-h light and 12-h dark.
Larvicidal Activity
Larvicidal activity of the aqueous crude extract and AgNPs from C. carandas was evaluated according to WHO [31]. The aqueous extract was tested at 80, 160, 240, 320 and 400 μg mL−1 concentrations and Ag NPs were tested at 7, 14, 21, 28 and 35 μg mL−1 concentrations. For each mosquito species, twenty late third instar larvae were introduced into a 500-mL glass beaker containing 250 mL of dechlorinated water, plus the desired concentrations of leaf extract or Ag NPs. For each concentration, five replicates were performed. Larval mortality was recorded at 24 h after exposure, during which no food was given to the larvae. Each bioassay included a control group (i.e. distilled water, no AgNP nor C. carandas leaf extract were added) with five replicates for each individual concentration.
Ovicidal Activity
To evaluate the ovicidal potential of the leaf axtract and AgNPs, the method of Su and Mulla [32] with slight modification of Govindarajan et al. [33] was followed. Eggs were collected from vector control laboratory, Department of Zoology, Annamalai University. The aqueous leaf extracts and silver nanoparticle were to achieve various concentrations ranging from 100 to 600 µg/ml and 10 to 60 µg/mL, respectively. Eggs of these mosquito species (n = 100 for 0–6, 6–12 and 12–18 h old egg rafts) were exposed to each concentration of leaf aqueous extract and AgNPs. After treatment, the eggs from each concentration were individually transferred to distilled water cups for hatching assessment after counting the eggs under a photomicroscope (Leica, Germany). Each experiment was replicated six times along with appropriate control. The hatch rates were assessed 48 h post-treatment by the following formula.
Biosafety on Non-target Mosquito Predators
The effect of non-target organisms was assessed following the method by Sivagnaname and Kalyanasundaram [34]. The effect of aqueous extract and Ag NPs of the potential plant was tested against non-target organisms A. bouvieri, D. indicus, and G. affinis. The species were field collected and separately maintained in cement tanks (85 cm diameter and 30 cm depth) containing water at 27 ± 3 °C and external R.H. 85 %. The aqueous extract and Ag NPs of C. carandas were evaluated at concentration of even 50 times higher if compared to the LC50 dose calculated for mosquito larvae. Ten replicates will be performed for each concentration along with four replicates of untreated controls. The non-target organisms were observed for mortality and other abnormalities such as sluggishness and reduced swimming activity after 48 h exposure. The exposed non-target organisms were also observed continuously for 10 days to understand the post treatment effect of this extract on survival and swimming activity.
Data Analysis
Larval mortality data were subjected to probit analysis. LC50 and LC90 were calculated using the method by Finney [35]. Data from ovicidal experiments were analyzed using ANOVA followed by Tukey's HSD test (p ≤ 0.05). In experiments evaluating biotoxicity on non-target organisms, the Suitability Index (SI) was calculated for each non-target species using the following formula [36].
All data were analyzed using the SPSS Statistical Software Package version 16.0. A probability level of P < 0.05 was used for the significance of differences between values.
Results and Discussion
Green-Synthesis and Characterization of Silver Nanoparticles
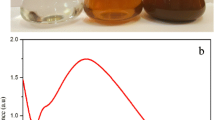
After incubation in dark room, the colorless reaction mixture turned into a dark brown solution, which indicated AgNPs synthesis (Fig. 1a). C. carandas-synthesized AgNPs exhibited a brown colour, due to excitation of surface Plasmon vibrations [18]. The surface Plasmon resonance bands are influenced by size, shape, morphology, composition and dielectric environment of prepared AgNPs [37]. Previous research has shown that spherical AgNPs contribute to the absorption band around 400-480 nm in the UV–vis spectrum [38]. These bands corresponded to that of AgNPs, and the UV–vis absorption spectrum showed broad surface plasmon resonance at 457.5 nm (Fig. 1b).
The crystalline nature of Ag NPs was studied by XRD analysis (Fig. 2a). To determine crystalline nature, size of nanoparticles and nature of the compounds involved in the stabilization of nanoparticles, XRD and FTIR studies were carried out. The obtained XRD patterns confirmed the crystalline nature of synthesized Ag NPs. Four diffraction peaks were observed at 38.5, 44.2, 65.1 and 78.4 represent the (111), (200), (220) and (311), reflections and the face-centered cubic structure of metallic silver, respectively. Our findings are in agreement with previous research conducted on Ag NPs green-synthesized using the leaf extract of S. acuta [15]. Spot energy-dispersive X-ray spectroscopy provides information on the composition at specific locations. Figure 2b, which is a representative profile of the spot EDX analysis, showed a strong signal in the silver region confirming the formation of Ag NPs; a distinct signal and high atomic percent values for silver were obtained [39].
FTIR spectroscopy was carried out to identify the possible biomolecules in C. carandas, which may be responsible for synthesis and stabilization of silver nanoparticles. Figure 3 shows that the FTIR spectrum of aqueous Ag NPs prepared from the C. carandas leaf extract showed main transmittance peaks at 3358.31, 1577.16, 1383.99, 1321.53, 1196.74, 1094.08, 1051.81, 753.91, 619.05 and 488.51 cm−1. The observed peaks denoted OH stretch, C=C bending, N–O stretch, C–N stretch, –H stretch, C–O stretch, and –OH groups, respectively. These bands denote stretching vibrational bands typical of compounds like flavonoids and terpenoids [40, 41] and may be responsible for efficient capping and stabilization of the obtained Ag NPs. Recently, it has been widely showed that the plant-mediated reduction of metallic nanoparticles is often due to terpenoids (citronellol and geraniol), flavones, ketones, aldehydes, amides, and carboxylic acids [42]. This suggests that the biological molecules may perform dual functions of reduction and stabilization of Ag NPs in the aqueous medium, possibly by in situ oxidation of hydroxyl groups and by the intrinsic carbonyl groups, as well as those produced by oxidation with air.
AFM is a primary tool for analyzing size, shape, agglomeration pattern and offers visualizations of three-dimensional views of the nanoparticles. It has an advantage over combination of high resolution, samples does not have to be conductive and does not require the high-pressure vacuum conditions [43]. 2.5 μm resolution studies of green-synthesized AgNPs with AFM reveal the particles are poly-dispersed, spherical in shape, having the size range from 1.6 to 7.4 nm and there is no agglomeration observed between the particles (Fig. 4a). Raw data obtained from AFM microscope were treated with a specially designed image processing software (NOVA-TX) to further exploit the 3D image of nanoparticles (Fig. 4b). The average particle size obtained from the corresponding diameter distribution was about 8 nm (Fig. 4c).
In addition, SEM and TEM was performed to investigate the morphology and size distribution of AgNPs (Fig. 5a, b). SEM showed that the morphology of AgNPs is mostly spherical, in agreement with the shape of SPR band in the UV–vis spectrum. Metallic Ag NPs generally show typical absorption peak approximately at 3 keV due to surface plasmon resonance [44]. TEM micrograph confirms the presence of poly-dispersed Ag NPs, showing fine configuration of crystalline, spherical AgNPs, with sizes sometimes higher if compared to AFM assays (e.g. 25-50 nm) (Fig. 5b). It was also noted that Ag NPs bound with thin layer of biomolecule coating on their surface which act as stabilizing agent, therefore Ag NPs were poly-dispersed without direct contact and stable for long period of time [45].
Acute Toxicity Against Mosquito Eggs and Larvae
In our experiments, both the C. carandas leaf extract and Ag NPs showed dose dependent larvicidal effect against all tested mosquito species (Tables 1 and 2). Compared to the leaf aqueous extract, green-synthesized Ag NPs showed higher toxicity against A. stephensi, A. aegypti, and C. quinquefasciatus with LC50 values of 14.33, 15.69 and 16.95 μg/mL, respectively (Table 2). The AgNPs exerted 100 % mortality (zero hatchability) when tested at 60 μg/mL. Control eggs showed 100 % egg hatchability (Tables 3, 4). In latest years, a growing number of evidences have been provided about the efficacy of plant-borne larvicides in the fight against mosquito vectors [7, 17, 46]. Combination of nanoparticles with bioactive principles bestows improved efficiency. The present study showed that the percentage of mosquito ovicidal and larvicidal mortality increased by about ten folds with the fabrication of bio-stabilized Ag NPs, over the C. carandas extract tested alone. Similarly, Govindarajan et al. [43] reported that the acute toxicity of Malva sylvestris leaf extract and green-synthesized Ag NPs were effective against larvae of An. stephensi, Ae. aegypti and Cx. quinquefasciatus. Compared to the leaf aqueous extract, Ag NPs showed higher toxicity against An. stephensi, Ae. aegypti, and Cx. quinquefasciatus with LC50 values of 10.33, 11.23, and 12.19 μg/mL, respectively. B. cristata-synthesized Ag NPs and aqueous leaf extract showed larvicidal properties against third instar larvae of the mosquito vectors An. subpictus, Ae. albopictus, and Cx. tritaeniorhynchus; LC50 values of synthesized Ag NPs were 12.46, 13.49, and 15.01 μg/mL, respectively and aqueous leaf extract LC50 values were 124.27, 135.32, and 146.31 μg/mL, respectively [16]. Moreover, Rajasekharreddy and Rani [47] determined that the Ag NPs produced using the seed extract of S. foetida showed mosquitocidal activity against IV instar larvae of Ae. aegypti (LC50 = 67.75 mg/ml), An. stephensi (LC50 = 57.36 mg/ml), and Cx. quinquefasciatus (LC50 = 71.54 mg/ml). Besides Aedes and Anopheles mosquitoes, low doses of Ag NPs were also toxic against other species, such as the filariasis mosquito Cx. quinquefasciatus. A good example is the toxic activity of C. scalpelliformis-synthesized Ag NPs, which had LC50 values from 3.08 ppm (I) to 7.33 ppm (pupae) [48]. Low doses of M. elengi-synthesized Ag NPs showed larvicidal and pupicidal toxicity against the malaria vector An. stephensi and the arbovirus vector Ae. albopictus. The LC50 value ranged from synthesized Ag NPs against An. stephensi, 12.53 (I) to 23.55 ppm (pupa) and LC50 against Ae. albopictus ranged from 11.72 ppm (I) to 21.46 ppm (pupa) [49]. Ovicidal activity of green-synthesized nanoparticles with S. muticum aqueous extract was toxic against An. stephensi, Ae. aegypti, and Cx. quinquefasciatus; the egg hatchability was reduced by 100 % after a single exposure to 30 ppm [50]. To our mind, the toxic action of AgNPs against mosquitoes may be attributed to the small size of the green-synthesized nanoparticles, which allows passage through the insect cuticle and into individual cells where they interfere with molting and other physiological processes [7].
Biosafety on Non-target Mosquito Predators
Concerning the biotoxicity of C. carandas aqueous extract and green-synthesized Ag NPs on non-target organisms A. bouvieri, D. indicus and G. affinis, results showed that the treatments achieved negligible toxicity against A. bouvieri, D. indicus and G. affinis, with LC50 values ranging from 1097.87 to 17249.89 µg/ml (Tables 5 and 6). Focal observations highlighted that longevity and swimming activity of the study species were not altered for at least a week after testing. SI indicated that C. carandas-fabricated AgNPs were less toxic to the non-target organism tested if compared to the targeted mosquito young instar populations (Table 7). Currently, moderate knowledge is available about the acute toxicity of mosquitocidal nanoparticles towards non-target aquatic species [7]. The biotoxicity of B. cristata aqueous extract and green-synthesized Ag NPs was evaluated on non-target organisms D. indicus, A. bouvieri, and G. affinis with LC50 values ranging from 633.26 to 8595.89 μg/mL, respectively [16]. B. tinctoria was tested against the non-target mosquito predators Toxorhynchites splendens and Mesocyclops thermocyclopoids, with LC50 values of 552.28 and 480.92 ppm, respectively. Experiments conducted testing Ag NPs on T. splendens and M. thermocyclopoids lead to LC50 values of 234.48 and 218.16 ppm, respectively. The SI calculated for the leaf extract of B. tinctoria was 3.02 and 2.63 for T. splendens and M. thermocyclopoids, respectively, while for Ag NPs, it was 47.1 and 43.8, respectively [51]. Recently, Govindarajan et al. [52] investigated the biotoxicity of C. spinarum aqueous extract and green-synthesized Ag NPs on non-target organisms D. indicus, A. bouvieri and G. affinis. Toxicity treatments achieved negligible toxicity against D. indicus, A. bouvieri and G. affinis, with LC50 values ranging from 424.09 to 6402.68 μg/mL. Furthermore, post-treatment with green-fabricated mosquitocidal nanoparticles, G. affinis showed higher predation rates against both An. stephensi and Ae. albopictus larvae. After 24 h, predation of III instar larvae of An. stephensi and Ae. albopictus were 60.90 and 57.42 %, respectively [49]. Also, Haldar et al. [46] did not detected toxicity of Ag NPs produced using dried green fruits of D. roxburghii against P. reticulata, after 48 h-exposure to LC50 of IV instar larvae of A. stephensi and C. quinquefasciatus. Mosquitocidal Ag NPs synthesized using Solanum nigrum berry extracts were not toxic against two mosquito predators, Toxorhynchites larvae and Diplonychus annulatum, and Chironomus circumdatus larvae, exposed to lethal concentrations of dry nanoparticles calculated on An. stephensi and Cx. quinquefasciatus larvae [53].
Conclusions
The one-pot plant-mediated fabrication of the Ag NPs is eco-friendly and cost-effective. In the present study, Ag NPs were rapidly biosynthesized at room temperature using a cheap C. carandas leaf extract. Bio-reduced silver nanocrystals were poly-dispersed and stable in solution for at least 4 weeks. Ag NPs have excellent anti-mosquitocidal activity against three important mosquito vectors. Our Ag NPs were mostly spherical in shape, crystalline in nature, with face-centered cubic geometry. This research highlighted that C. carandas-synthesized Ag NPs are easy to produce, stable over time, and can be employed at low dosages to strongly reduce populations of vectors mosquitoes without detrimental effects on predation rates of non-target aquatic organisms, such as A. bouvieri, D. indicus, and G. affinis.
References
G. Benelli and H. Mehlhorn (2016). Parasitol. Res.. doi:10.1007/s00436-016-4971-z.
G. Benelli (2015). Parasitol. Res. 114, 3201.
A. F. Howard, G. Zhou, and F. X. Omlin (2007). BMC Public Health 7, 199.
K. Raghvendra, S. K. Subbarao (2002). ICMR Bull. 32, 93.
M. Sarwar, N. Ahmad, and M. Toufiq (2009). Pak. J. Bot. 41, 3047.
G. Benelli (2015). Parasitol. Res. 114, 2801.
G. Benelli (2016). Parasitol. Res.. doi:10.1007/s00436-015-4800-9.
R. Pavela (2015). Ind. Crops Prod. 76, 174.
R. Pavela (2015). Ind. Crops Prod. 30, 311.
U. Muthukumaran, M. Govindarajan, and M. Rajeswary (2015). Parasitol. Res. 114, 1817.
M. Zhang, M. Liu, H. Prest, and S. Fischer (2008). Nano Lett. 8, 1277.
S. H. Jeong, S. Y. Yeo, and S. C. Yi (2005). J. Mater. Sci. 40, 5407.
N. Savithramma, R. M. Linga, K. Rukmini, and D. P. Suvarnalatha (2011). Int. J. ChemTech. Res. 3, 1394.
A. Saxena, R. M. Tripathi, and R. P. Singh (2010). Dig. J. Nanomater. Biostruct. 5, 427.
K. Veerekumar, M. Govindarajan, and M. Rajeswary (2013). Parasitol. Res. 112, 4073–4085.
M. Govindarajan and G. Benelli (2016). Parasitol. Res. 115, 925.
U. Muthukumaran, M. Govindarajan, and M. Rajeswary (2015). Parasitol. Res. 114, 989.
K. Veerakumar, M. Govindarajan, M. Rajeswary, and U. Muthukumaran (2014). Parasitol. Res. 113, 1775.
K. Veerakumar, M. Govindarajan, M. Rajeswary, and U. Muthukumaran (2014). Parasitol. Res. 113, 2363.
M. Govindarajan, M. Rajeswary, S. L. Hoti, K. Murugan, K. Kovendan, S. Arivoli, and G. Benelli (2016). J. Asia Pac. Entomol. 19, 51.
M. Govindarajan, M. Rajeswary, K. Veerakumar, U. Muthukumaran, S. L. Hoti, H. Mehlhorn, D. R. Barnard, and G. Benelli (2016). Parasitol. Res. 115, 723.
M. Govindarajan, M. Rajeswary, K. Veerakumar, U. Muthukumaran, S. L. Hoti, and G. Benelli (2016). Exp. Parasitol. 161, 40.
C. D. Patil, S. V. Patil, H. P. Borase, B. K. Salunke, and R. B. Salunkhe (2012). Parasitol. Res. 110, 1815.
G. Benelli, A. Lo Iacono, A. Canale, and H. Mehlhorn (2016). Parasitol. Res.. doi:10.1007/s00436-016-5037-y.
C. P. Khare Indian Medicinal Plants (An Illustrated Dictionary) (Springer Science and Business Media, New York, 2007), p. 472.
K. R. Kirtikar and B. D. Basu Indian Medicinal Plants, vol. II (Lalit Mohan Basu, Allahabad, 2003), pp. 1546–1549.
B. N. Dhawan and G. K. Patnaik (1985). Indian Drugs 22, 285.
R. S. L. Taylor, J. B. Hudson, N. P. Manandhar, and G. H. N. Tower (1996). J. Ethnopharmacol. 53, 97.
R. C. Rastogi, M. M. Vohra, R. P. Rastogi, and M. L. Dhar (1996). Indian J. Chem. 4, 132.
M. Govindarajan and R. Sivakumar (2015). Parasitol. Res. 114, 601.
World Health Organization (2005). WHO, Geneva, HO/CDS/WHOPES/GCDPP/1.3.
T. Su and M. S. Mulla (1998). J. Am. Mosq. Control Assoc. 14, 204.
M. Govindarajan, A. Jebanesan, and T. Pushpanathan (2008). Parasitol. Res. 102, 289.
N. Sivagnaname and M. Kalyanasundaram (2004). Mem. Inst. Oswaldo Cruz Rio. De. Janeiro 99, 115.
D. J. Finney Probit Analysis (Cambridge University Press, London, 1971), pp. 68–72.
P. G. Deo, S. B. Hasan, and S. K. Majumdar (1988). Int. Pest Control. 30, 118.
K. L. Kelly, E. Coronado, L. L. Zhao, and G. C. Schatz (2003). J. Phys. Chem. B 107, 668.
K. Shameli, M. B. Ahmad, S. D. Jazayeri, P. Shabanzadeh, P. Sangpour, H. Jahangirian, and Y. Gharayebi (2012). Chem. Cent. J. 6, 73.
A. M. Fayaz, K. Balaji, M. Girilal, R. Yadav, P. T. Kalaichelvan, and R. Venketesan (2010). Nanomed. Nanotechnol. Biol. Med. 6, e103.
B. S. Siddiqui, F. Afshan, G. S. Faizi, S. N. H. Naqvi, and R. M. Tariq (2000). Phytochemistry 53, 371.
J. Huang, Q. Li, and D. Sun (2007). Nanotechnology 18, 105.
S. S. Shankar, A. Rai, A. Ahmad, and M. Sastry (2004). J. Colloid Interface Sci. 275, 496.
M. Govindarajan, S. L. Hoti, M. Rajeswary, and G. Benelli (2016). Parasitol. Res.. doi:10.1007/s00436-016-5038-x.
P. Magudapatty, P. Gangopadhyayransm, B. K. Panigrahi, K. G. M. Nair, and S. Dhara (2001). Phys. B 299, 142.
V. Vignesh, K. F. Anbarasi, S. Karthikeyeni, G. Sathiyanarayanan, P. Subramaniana, and R. Thirumurugan (2013). Colloid Surf. A 439, 184.
K. M. Haldar, B. Haldar, and G. Chandra (2013). Parasitol. Res. 112, 1451.
P. Rajasekharreddy and P. Usha Rani (2014). Mater. Sci. Eng. C 39, 203.
K. Murugan, G. Benelli, C. Panneerselvam, J. Subramaniam, T. Jeyalalitha, D. Dinesh, M. Nicoletti, J. S. Hwang, U. Suresh, and P. Madhiyazhagan (2015). Exp. Parasitol. 153, 129.
J. Subramaniam, K. Murugan, C. Panneerselvam, K. Kovendan, P. Madhiyazhagan, P. Mahesh Kumar, D. Dinesh, B. Chandramohan, U. Suresh, M. Nicoletti, A. Higuchi, J. S. Hwang, S. Kumar, A. A. Alarfaj, M. A. Munusamy, R. H. Messing, and G. Benelli (2015). Environ. Sci. Pollut. Res. 22, 20067.
P. Madhiyazhagan, K. Murugan, A. N. Kumar, T. Nataraj, D. Dinesh, C. Panneerselvam, J. Subramaniam, P. Mahesh Kumar, U. Suresh, M. Roni, M. Nicoletti, A. A. Alarfaj, A. Higuchi, M. A. Munusamy, and G. Benelli (2015). Parasitol. Res. 114, 4305.
P. Mahesh Kumar, K. Murugan, P. Madhiyazhagan, K. Kovendan, D. Amerasan, B. Chandramohan, D. Dinesh, U. Suresh, M. Nicoletti, M. Saleh Alsalhi, S. Devanesan, H. Wei, K. Kalimuthu, J. S. Hwang, A. Lo Iacono, and G. Benelli (2016). Parasitol. Res. 115, 751.
M. Govindarajan, M. Nicoletti, and G. Benelli (2016). J. Clust. Sci. 27, 745.
A. Rawani, A. Ghosh, and G. Chandra (2013). Acta Trop. 128, 613.
Acknowledgments
Professor Charles M. Lukehart and two anonymous reviewers improve an earlier version of our manuscript. The authors would like to thank Professor and Head, Department of Zoology, Annamalai University for the laboratory facilities provided. The authors would also like to acknowledge the cooperation of staff members of the VCRC (ICMR), Pondicherry and thankful to Dr. S. Ramesh, Professor and Head, Veterinary college, Vepery, Chennai for TEM analysis.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
The Authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Govindarajan, M., Benelli, G. A Facile One-Pot Synthesis of Eco-Friendly Nanoparticles Using Carissa carandas: Ovicidal and Larvicidal Potential on Malaria, Dengue and Filariasis Mosquito Vectors. J Clust Sci 28, 15–36 (2017). https://doi.org/10.1007/s10876-016-1035-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-016-1035-6