Abstract
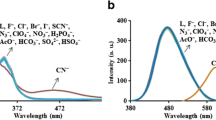
We developed a new spectrofluorometric method for qualitative and quantitative determination of cyanide in water using the incorporation of naphthoquinone imidazole boronic-based sensors (m -NQB and p -NQB) and a cationic surfactant, certyltrimethyl ammonium bromide (CTAB). This micellar system exhibited great selectivity for cyanide detection with an assistance of the cationic surface of micelle. The interaction of boronic acid of the sensor toward cyanide in CTAB micellar media gave a quantitative measure of cyanide concentration in the micromolar level. Under the optimal condition, fluorescence intensity at 460 nm of m -NQB and p -NQB provided two sets of linear ranges, 0.5–15 μM and 20–40 μM and the limit of cyanide detection of 1.4 μM. Hence, both sensors in CTAB aqueous micellar system offered a considerably promising cyanide detection with 1000–fold enhancement of the detection limit compared to those studied in DMSO: H2O. The proposed sensors could also be used to determine cyanide in water with good analytical characteristics.











Similar content being viewed by others

References
Guidelines for Drinking-Water Quality. World Health Organization, Geneva, (1996)
Ishii A, Watanabe-Suzuki H, Suzuki O, Kumazawa T (1998) Determination of cyanide in whole blood by capillary gas chromatography with cryogenic oven trapping. Anal Chem 70:4873–4876
González LaFuente JM, Fernández Martínez E, Vicente Pérez JA, Fernández Fernández S, Miranda Ordiores AJ, Sánchez Uría JE, Fernández Sánchez ML, Sanz-Medel A (2000) Differential-pulse voltammetric determination of low μgl−1 cyanide levels using EDTA, Cu(II) and a hanging mercury drop electrode. Anal Chim Acta 410:135–142
Vallejo-Pecharromán B, Luque de Castro MD (2002) Determination of cyanide by a pervaporation–UV photodissociation–potentiometric detection approach. Analyst 127:267–270
López Gómez AV, Martínez Calatayud J (1998) Determination of cyanide by a flow injection analysis-atomic absorption spectrometric method. Analyst 123:2103–2107
Miralles E, Prat D, Compañó R, Granados M (1997) Assessment of different fluorimetric reactions for cyanide determination in flow systems. Analyst 122:553–558
Recalde-Ruiz DL, Andrés-García E, Díaz-García ME (2000) Fluorimetric flow injection and flow-through sensing systems for cyanide control in waste water. Analyst 125:2100–2105
Miralles E, Compañó R, Granados M, Prat MD (2000) Determination of metal-cyanide complexes by ion-interaction chromatography with fluorimetric detection. Anal Chim Acta 403:197–204
Gamoh K, Imamichi S (1991) Postcolumn liquid chromatographic method for the determination of cyanide with fluorimetric detection. Anal Chim Acta 251:255–259
Miyaji H, Sessler JL (2001) Off-the-shelf colorimetric anion sensors. Angrew Chem Int Ed 40:154–157
Jr Anzenbacher P, Tyson DS, Jursíková K, Castellano FN (2002) Luminescence lifetime-based sensor for cyanide and related anions. J Am Chem Soc 124:6232–6233
Kim Y-H, Hong J-I (2002) Ion pair recognition by Zn–porphyrin/crown ether conjugates: visible sensing of sodium cyanide. Chem Commun 512–513
Chow C-F, Lam MHW, Wong W-Y (2004) A heterobimetallic ruthenium(II)-copper(II) Donor-acceptor complex as a chemodosimetric ensemble for selective cyanide detection. Inorg Chem 43:8387–8393
Chung S-Y, Nam S-W, Lim J, Park S, Yoon J (2009) A highly selective cyanide sensing in water via fluorescence change and its application to in vivo imaging. Chem Commun 2866–2868
Chung Y, Lee H, Ahn KH (2006) N-acyl triazenes as tunable and selective chemodosimeters toward cyanide Io. J Org Chem 71:9470–9474
Chen C-L, Chen Y-H, Chen C-Y, Sun S-S (2006) Dipyrrole carboxamide derived selective ratiometric probes for cyanide ion. Org Lett 8:5053–5056
Tomosulo M, Sortino S, White AJP, Raymo FM (2006) Chromogenic oxazines for cyanide detection. J Org Chem 71:744–753
Lee K-S, Kim H-J, Shin I, Hong J-I (2008) Fluorescence chemodosimeter for selective detection of cyanide in water. Org Lett 10:49–51
Ekmekci Z, Yilmaz MD, Akkaya EU (2008) A monostyryl-boradiazaindacene (BODIPY) derivative as colorimetric and fluorescence probe for cyanide ions. Org Lett 10:461–464
Yang Y-K, Tae J (2006) Acridinium salt based fluorescence and colorimetric chemosensor for the detection of cyanide in water. Org Lett 8:5721–5723
Ros-Lis JV, Matinez-Manez R, Soto J (2002) A selective chromogenic reagent for cyanide determination. Chem Commun 2248–2249
Hudnall TW, Gabbaï FP (2007) Ammonium boranes for the selective complexation of cyanide or fluoride ions in water. J Am Chem Soc 129:11978–11986
Sun Y, Wang G, Guo W (2009) Colorimetric detection of cyanide with N-nitrophenyl benzamide derivatives. Tetrahedron 65:3480–3485
Huh JO, Do Y, Lee MH (2008) A BODIPY-Borane dyad for the selective complexation of cyanide ion. Organometallics 27:1022–1025
Badugu R, Lakowicz JR, Geddes CD (2005) Anion sensing using quinolinium based boronic acid probes. Curr Anal Chem 1:157–170
Badugu R, Lakowicz JR, Geddes CD (2005) Cyanide-sensitive fluorescence probes. Dyes Pigments 64:49–55
Badugu R, Lakowicz JR, Geddes CD (2005) Enhanced fluorescence cyanide detection at physiologically lethal levels: reduced ICT-based signal transduction. J Am Chem Soc 127:3635–3641
Badugu R, Lakowicz JR, Geddes CD (2004) Fluorescence intensity and lifetime-based cyanide sensitive probes for physiological safeguard. Anal Chem Acta 522:9–17
Jamkratoke M, Ruangpornvisuti V, Tumcharern G, Tuntulani T, Tomapatanaget B (2009) A-D-A sensors based on naphthoimidazoledione and boronic acid as Turn-On cyanide probes in water. J Org Chem 74:3919–3922
Fernandez YD, Gramateges AP, Amendola V, Foti F, Mangano C, Pallavicini P, Patroni S (2004) Using micelles for a new approach to fluorescence sensors for metal cations. Chem Commun 1650–1651
Nakahara Y, Kida T, Nakatsuji Y, Akashi M (2004) A novel fluorescence indicator for Ba2+ in aqueous micellar solutions. Chem Commun 224–225
Nakahara Y, Kida T, Nakatsuji Y, Akashi M (2005) Fluorometric sensing of alkali metal and alkaline earth metal cations by novel photosensitive monoazacryptand derivatives in aqueous micellar solutions. Org Biomol Chem 3:1787–1794
Vargas LV, Sand J, Brãno TAS, Fiedler HD, Quina FH (2005) Determination of environmentally important metal ions by fluorescence quenching in anionic micellar solution. Analyst 130:242–246
Mallick A, Mandal MC, Haldar B, Charabarty A, Das P, Chattopadhyay N (2006) Surfactant-induced modulation of fluorosensor activity: a simple way to maximize the sensor efficiency. J Am Chem Soc 126:3126–3127
Pallavicini P, Dias-Fernandez YA, Foti F, Mangano C, Patroni S (2007) Fluorescence sensors for Hg2+ in micelles: a new approach that transforms an ON–OFF into an OFF–ON response as a function of the lipophilicity of the receptor. Chem Eur J 13:178–187
Cuccovia IM, Chaimovich H (1982) Determination of micromolar concentrations of iodine with aqueous mice1lar hexadecyltrimethylammonium bromide. Anal Chem 54:789–791
Kunda S, Ghosh SK, Manadal M, Pal T (2002) Micelle bound redox dye marker for nanogram level arsenic detection promoted by nanoparticles. New J Chem 26:1081–1084
Hayakawa K, Kanda M, Satake I (1979) The determination of formation constant of triiodide ion in micellar solution of dodecyltrimethyammonium choloride. Bull Chem Jpn Soc 52:3171–3175
Grosh SK, Kundu S, Mandal M, Pal T (2002) Silver and gold nanocluster catalyzed reduction of methylene blue by arsine in a micellar medium. Langmuir 18:8756–8760
Button CA, Nome F, Quina FH, Romsted LS (1991) Ion binding and reactivity at charged aqueous interfaces. Acc Chem Res 24:357–364
Mallick K, Jewraka S, Pradhan N, Pal T (2001) Micelle-catalysed redox reaction. Curr Sci 80:1408–1412
Matizinger S, Hussey DM, Fayer MD (1998) Fluorescence probes solubilization in the headgroup and core regions of micelles: fluorescence lifetime and orientational relaxation measurement. J Phy Chem B 102:7216–7224
Wei L, Ming Z, Jinli Z, Yongcai H (2006) Self-assembly of cetyl trimethylammonium bromide in ethanol-water mixtures. Front Chem China 4:438–442
Cooper CR, Spencer N, James TD (1998) Selective fluorescence detection of fluoride using boronic acids. Chem Commun 1365–1366
Ingle JD Jr, Wilson RL (1976) Difficulties with determining the detection limit with nonlinear calibration curves in spectrometry. Anal Chem 48:1641–1642
Acknowledgements
M.J. is a Ph.D student supported by the Royal Golden Jubilee Program (PHD/0049/2550) of the Thailand Research Fund (TRF) and Commission on Higher Education (CHE). We gratefully acknowledge the National Nanotechnology Center (NN-B-22-b15-94-49-55), the TRF and CHE (RTA5080006) and Project for Establishment of Comprehensive Center for Innovative Food, Health Products and Agriculture (PERFECTA) for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jamkratoke, M., Tumcharern, G., Tuntulani, T. et al. A Selective Spectrofluorometric Determination of Micromolar Level of Cyanide in Water Using Naphthoquinone Imidazole Boronic-Based Sensors and a Surfactant Cationic CTAB Micellar System. J Fluoresc 21, 1179–1187 (2011). https://doi.org/10.1007/s10895-010-0796-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-010-0796-9