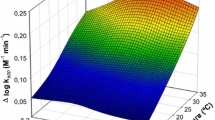
The oxidation rates of nanomolar levels of Fe(II) in seawater (salinity S = 36.2) by mixtures of O2 and H2O2 has been measured as a function of pH (5.8–8.4) and temperature (3–35∘C). A competition exists for the oxidation of Fe(II) in the presence of both O2 (μ mol⋅L−1 levels) and H2O2 (nmol⋅L−1 levels). A kinetic model has been applied to explain the experimental results that considers the interactions of Fe(II) with the major ions in seawater. In the presence of both oxidants, the hydrolyzed Fe(II) species dominate the Fe(II) oxidation process between pH 6 and 8.5. Over pH range 6.2–7.9, the FeOH+ species are the most active, whereas above pH 7.9, the Fe(OH)02 species are the most active at the levels of CO2−3 concentration present in seawater. The predicted Fe(II) oxidation rate at [Fe(II)]0 = 30nmol⋅L−1 and pH = 8.17 in the oxygen-saturated seawater with [H2O2]0 = 50nmol⋅L−1 (log 10 k = −2.24s−1) is in excellent agreement with the experimental value of log 10 k = −2.29s−1 ([H2O2]0 = 55nmol⋅L−1, pH = 8).
Similar content being viewed by others
References
R. G. Pestane and R. G. Zika, Fate of Superoxide in Coastal Seawater, Nature 325, 516–518 (1987).
R. G. Zika, J. W. Moffett, R. G. Pestane, W. J. Cooper, and E. S. Saltzman, Spatial and Temporal Variations of Hydrogen Peroxide in Gulf of Mexico Waters, Geochim. Cosmochim. Acta 49, 1173–1184 (1985).
C. A. Moore, C. T. Farmer, and R. G. Zika, Influence of the Orinoco River on Hydrogen Peroxide Distribution and Production in the Eastern Caribbean, J. Geophys. Res. 98, 2289–2298 (1993).
J. W. Moffett and R. G. Zika, Reaction Kinetics of Hydrogen Peroxide with Copper and Iron in Seawater, Environ. Sci. Technol. 21, 804–810 (1987).
D. W. King, H. A. Lounsbury, and F. J. Millero, Rates and Mechanism of Fe(II) Oxidation at Nanomolar Total Iron Concentrations, Environ. Sci. Technol. 29, 818–824 (1995).
J. M. Santana-Casiano, M. González-Dávila, and F. J. Millero, Oxidation of Nanomolar Levels of Iron(II) with Oxygen in Seawater, Environ. Sci. Technol. 39, 2073–2079 (2005).
A. L. Rose and T. D. Waite, Kinetic Model for Fe(II) Oxidation in Seawater in the Absence and Presence of Natural Organic Matter, Environ. Sci. Technol. 36, 433–444 (2002).
K. W. Bruland and E. L. Rue, Analytical Methods for the Determination of Concentrations and Speciation of Iron, in The Biogeochemistry of Iron in Seawater, D. R. Turner and K. A. Hunter, Eds. (Wiley, England, 2001), pp. 255–289.
K. H. Coale, K. S. Johnson, S. E. Fitzwater, S. P. G. Blain, T. P. Stanton, and T. L. Coley, IronEx-1, an In Situ Iron-enrichment Experiment: Experimental Design, Implementation and Results, Deep Sea Res. II 45, 919–945 (1998).
P. L. Croot, P. Laan, J. Nishioka, V Strass, B. Cisewski, M. Boye, K. R. Timmermans, R. G. Bellerby, L. Goldson, P. Nightigale, and H. J. W de Baar, Spatial and Temporal Distribution of Fe(II) and H2O2 During EisenEX, an Open Ocean Mesoscale Iron Enrichment, Mar. Chem. 95, 65–88 (2005).
M. González-Dávila, J. M. Santana-Casiano, and F. J. Millero, Oxidation of Nanomolar Levels of Iron(II) with H2O2 in Seawater, Geochim. Cosmochim. Acta 69, 83–93 (2005).
F. J. Millero, The pH of Estuarine Waters, Limnol. Oceanogr. 31, 839–847 (1986).
E. Viollier, P. W. Inglet, K. Hunter, A. N. Roychuodhury, and P. Capellen, The Ferrozine Method Revisited: Fe(II)(Fe(III) Determination in Natural Waters, App. Geochem. 15, 785–790 (2000).
J. Z. Zhang, C. Kelble, and F. J. Millero, Gas-segmented Continuous Flow Analysis of Iron in Water with a Long Liquid Waveguide Capillary Flow Cell, Anal. Chim. Acta. 438, 49–57 (2001).
R. G. Zika and E. S. Saltzman, Interaction of Ozone and Hydrogen Peroxide in Water: Implication for Analysis of H2O2 in Air, Geophys. Res. Lett. 9, 231–234 (1982).
H. P. Hansen, Determination of Oxygen, in Methods of Seawater Analysis, K. Grasshoff, K. Kremling, and M. Ehrhardt, Eds. (Wiley-VCH, Germany, 1999), pp. 75–89, Chapter 4.
F. J. Millero, S. Sotolongo, and M. Izaguirre, The Kinetics of Oxidation of Fe(II) in Seawater, Geochim. Cosmochim. Acta 51, 793–801 (1987).
W. Stumm and G. F. Lee, Kinetic Product of Ferrous Iron, Ind. Eng. Chem. 53, 143–146 (1961).
F. J. Millero and S. Sotolongo, The Oxidation of Fe(II) with H2O2 in Seawater, Mar. Chem. 53, 1867–1873 (1989).
D. W. King and R. Farlow, Role of Carbonate Speciation on the Oxidation of Fe(II) by H2O2, Mar. Chem. 70, 201–209 (2000).
J. M. Santana-Casiano, M. González-Dávila, and F. J. Millero, The Oxidation of Fe(II) in NaCl–HCO3 and Seawater Solutions in the Presence of Phthalate and Salicylate Ions: A Kinetic Model, Mar. Chem. 85, 27–40 (2004).
D. W. King, Role of Carbonate Speciation on the Oxidation Rate of Fe(II) in Aquatic Systems, Environ. Sci. Technol. 32, 2997–3003 (1998).
J. D. Rush and B. H. J. Bielsky, Pulse Radiolytic Studies of the Reactions of HO2/O2− with Fe(II)/Fe(III) Ions. The Reactivity of HO2/O2− with Ferric Ions and its Implication on the Occurrence of the Haber–Weiss Reaction, J. Phys. Chem. 89, 5062–5066 (1985).
B. M. Voelker, D. L. Sedlak, and O. Zafiriou, Chemistry of Superoxide Radical in Seawater: Reaction with Organic Cu Complexes, Environ. Sci. Technol. 34, 1036–1042 (2000).
T. L. Theis and P. C. Singer, Complexation of Fe(II) with Organic Matter and its Effect on Fe(II) Oxygenation, Environ. Sci. Technol. 8, 569–573 (1974).
J. M. Santana-Casiano, M. González-Dávila, M. J. Rodríguez, and F. J. Millero, The Effects of Organic Compounds in the Oxidation Kinetics of Fe(II), Mar. Chem. 70, 211–222 (2000).
B. M. Voelker and B. Sulzberger, Effects of Fulvic Acid on Fe(II) Oxidation by Hydrogen Peroxide, Environ. Sci. Technol. 30, 1106–1114 (1996).
A. L. Rose and T. D. Waite, Effect of Dissolved Natural Organic Matter on the Kinetics of Ferrous Iron Oxygenation in Seawater, Environ. Sci. Technol. 37, 4877–4886 (2003).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
González-Dávila, M., Santana-Casiano, J.M. & Millero, F.J. Competition Between O2 and H2O2 in the Oxidation of Fe(II) in Natural Waters. J Solution Chem 35, 95–111 (2006). https://doi.org/10.1007/s10953-006-8942-3
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10953-006-8942-3