Abstract
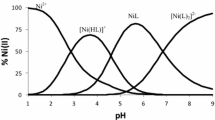
In this paper we report the formation of binary and ternary nickel(II) complexes involving dipicolinic acid (H2Dipic) as the primary ligand and some selected amino acids {glycine (HGly), ⍺-alanine (H⍺-Ala), β-alanine (Hβ-Ala) and proline (HPro)} as secondary ligands. These complexes were studied at 25 °C by means of electromotive force measurements, emf(H), using 1.0 mol⋅dm−3 NaCl as the ionic medium. The experimental data were analyzed by means of the computational least-squares program LETAGROP, taking into account hydrolysis of the nickel(II) cation and the acid/base reactions of the ligands whose equilibrium constants were kept fixed during the analysis. In the study of the binary nickel(II)–amino acids systems the species [NiL]+, NiL2 and [NiL3]− were observed, and in the case of the ternary nickel(II)–dipicolinic acid–amino acids systems the complexes Ni(Dipic)HL, [Ni(Dipic)L]− and [Ni(Dipic)L(OH)]2− were observed. The respective stability constants were determined, and the species distribution diagrams, as a function of pH, are briefly discussed.










Similar content being viewed by others
References
Idriss, K.A., Saleh, M.S., Sedaira, H., Seleim, M.M., Hashem, E.Y.: Solution equilibria and stability of the complexes of pyridinecarboxylic acids: complexation reaction of mercury(II) with 2-hydroxynicotinic acid. Monatsh. Chem. 122, 507–520 (1991)
Erikson, T.E., Grenthe, I., Puigdomenech, I.: A kinetic investigation of lanthanide(III) complex formation with picolinic acid. Inorg. Chim. Acta 126, 131–135 (1987)
Ducommun, Y., Helm, L., Laurenezy, G., Merbach, A.: Variable pressure spectrophotometric equilibrium and 139La NMR kinetic studies of lanthanum(III) ion complex formation with 2,6-dicarboxy-4-hydroxypyridine in aqueous solution. Inorg. Chim. Acta 158, 3–4 (1989)
Yasumatsu, N., Yoshikawa, Y., Adachi, Y., Sakurai, H.: Antidiabetic copper(II)–picolinate: impact of the first transition metal in the metallopicolinate complexes. Bioorg. Med. Chem. 15, 4917–4922 (2007)
Fukui, K., Fujisawa, Y., Ohya-Nishiguchi, H., Kamada, H., Sakurai, H.: In vivo coordination structural changes of a potent insulin-mimetic agent, bis(picolinato)oxovanadium(IV), studied by electron spin-echo envelope modulation spectroscopy. J. Inorg. Biochem. 77, 215–224 (1999)
Kolthoff, I.M., Stenger, V.A.: Volumetric Analysis, vol. II, p. 94. Interscience, New York (1947)
Jeffery, G.H., Bassett, J., Mendham, J., Denney, R.C.: Vogel’s Textbook of Quantitative Chemical Analysis, 5th edn., p. 327. Longman Scientific and Technical, Essex (1989)
Gran, G.: Determination of the equivalence point in potentiometric titrations. Part II. Analyst 77, 661–671 (1952)
Biedermann, G., Sillén, L.G.: The hydrolysis of metal ions. IV. Liquid-junction potentials and constancy of activity factors in NaClO4–HClO4 ionic medium. Ark. Kemi 5, 425–540 (1953)
Brito, F., Araujo, M.L., Lubes, V., D’Ascoli, A., Mederos, A., Gili, P., Dominguez, S., Chinea, E., Hernandez, R., Armas, M.T., Baran, E.: EMF(H) data analysis of weak metallic complexes using reduced formation functions. J. Coord. Chem. 58, 501–512 (2005)
Sillén, L.G., Warnqvist, B.: High-speed computers as a supplement to graphical methods. VI. A strategy for two-level Letagrop adjustment of common and “group” parameters. Features that avoid divergence. Ark. Kemi 31, 315–339 (1969)
Veliz, L., Martínez, J.D., Araujo, M.L., Brito, F., Lubes, G., Rodríguez, M., Lubes, V.: Estudio de la hidrólisis del ion Niquel(II) y de la formación de los complejos de Niquel(II) con los ácidos picolínico y dipicolínico en NaCl 1,0 mol⋅dm−3 a 25 °C. Avances Quím. 6(1), 3–8 (2011)
Alderighi, L., Gans, P., Ienco, A., Peters, D., Sabatini, A., Vacca, A.: Hyperquad simulation and speciation (HySS): a utility program for the investigation of equilibria involving soluble and partially soluble species. Coord. Chem. Rev. 184, 311–318 (1999)
Martell, A.E., Smith, M., Motekaitis, R.J.: NIST critical stability constants of metal complexes database. U.S. Department of Commerce, Gaithersburg, MD (1993)
Powell, K.J., Pettit, L.D.: IUPAC stability constants database. Academic Software, Otley (U.K.) (1997)
Khalil, M.M., Attia, A.E.: Potentiometric studies on the binary and ternary complexes of copper(II) containing dipicolinic acid and amino acids. J. Chem. Eng. Data 44, 180–184 (1999)
Acknowledgements
We acknowledge the Decanato de Investigación y Desarrollo (DID) from Simón Bolívar University. We acknowledge also Professors Antonio Barriola, Antonio Zapata, and Simón López from the Simón Bolívar University for their help during this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hernández, R., Rodríguez, R., Martínez, J.D. et al. Complexation Equilibria and Determination of Stability Constants of Binary and Ternary Nickel(II) Complexes with Amino Acids (Glycine, α-Alanine, β-Alanine and Proline) and Dipicolinic Acid as Ligands. J Solution Chem 41, 1103–1111 (2012). https://doi.org/10.1007/s10953-012-9867-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-012-9867-7