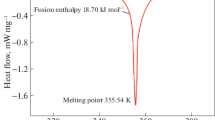
Abstract
The solubility data of sorbic acid in binary systems of (ethanol + water), (1-propanol + water) and (2-propanol + water) were measured from 283.15 to 323.15 K using the static equilibrium method under atmospheric pressure. It was found that the solubility of sorbic acid in the three binary solvent systems increased with increasing temperature as well as increasing initial mole fraction of organic solvent in these systems. The van’t Hoff–Jouyban–Acree model, the modified Apelblat–Jouyban–Acree model and the CNIBS/R-K model were proposed for correlating the experimental solubility values in various solution systems. Furthermore, the dissolution thermodynamic properties of Gibbs energy change (ΔsolGo), molar enthalpy change (ΔsolHo) and molar entropy change (ΔsolSo) were calculated from the experimental solubility data, using the van’t Hoff equation. The positive values of ΔsolGo, ΔsolHo and ΔsolSo indicate that these dissolution processes of sorbic acid in the solvents studied were all endothermic and entropically favorable. In addition, the change of dissolution enthalpy was the main contributor to the positive value of the molar Gibbs energy of the dissolution process. The experimental solubility and the models used in this work would be conducive to purifying sorbic acid from its crude mixtures.






Similar content being viewed by others
References
Smith, D.P., Rollin, N.J.: Sorbic acid as a fungistatic agent for foods. J. Food Sci. 19(1–6), 59–65 (2006)
Bell, T.A., Etchells, J.L., Borg, A.F.: Influence of sorbic of acid on the growth of certain species of bacteria, yeasts, and filamentous fungi. J. Bacteriol. 77, 573–580 (1959)
Tzatzarakis, M.N., Tsatsakis, A.M., Lotter, M.M., Shtilman, M.I., Vakalounakis, D.J.: Effect of novel water-soluble polymeric forms of sorbic acid against Fusarium oxysporum f. sp. radicis-cucumerinum. Food Addit. Contamin. 17(12), 965–971 (2000)
Eklund, T.: The antimicrobial effect of dissociated and undissociated sorbic acid at different pH levels. J. Appl. Bacteriol. 54(3), 383–389 (1983)
Stratford, M., Anslow, P.A.: Evidence that sorbic acid does not inhibit yeast as a classic ‘weak acid preservative’. Lett. Appl. Microbiol. 27(4), 203–206 (1998)
Wang, J., Xu, R., Xu, A.: Solubility determination and thermodynamic functions of 2-chlorophenothiazine in nine organic solvents from T = 283.15 K to T = 318.1 5 K and mixing properties of solutions. J. Chem. Thermodyn. 106, 132–144 (2017)
Fang, J., Zhang, M., Zhu, P., Ouyang, J., Gong, J., Chen, W., Xu, F.: Solubility and solution thermodynamics of sorbic acid in eight pure organic solvents. J. Chem. Thermodyn. 85, 202–209 (2015)
Zhao, H., Xu, H., Yang, Z., Li, R.: Solubility of 3,4-dichloronitrobenzene in methanol, ethanol, and liquid mixtures (methanol plus water, ethanol plus water): experimental measurement and thermodynamic modeling. J. Chem. Eng. Data 58(11), 3061–3068 (2013)
Xu, H., Zhang, B., Yang, Z., Yao, G., Zhao, H.: Solubility of dichloronitrobenzene in eight organic solvents from T = (278.15 to 303.15) K: measurement and thermodynamic modeling. J. Chem. Eng. Data. 59(4), 1281–1287 (2014)
Jiang, X., Hu, Y., Meng, Z., Yang, W., Shen, F.: Solubility of succinic acid in different aqueous solvent mixtures: experimental measurement and thermodynamic modeling. Fluid Phase Equil. 341, 7–11 (2013)
Ferreira, L.A., Macedo, E.A., Pinho, S.P.: Solubility of amino acids and diglycine in aqueous-alkanol solutions. Chem. Eng. Sci. 59(15), 3117–3124 (2004)
Tan, Q., Leng, Y., Wang, J., Huang, C., Yuan, Y.: Correlation and prediction of the solubility of the racemic tartaric acid–ethanol–water system with the NRTL model. J. Mol. Liquids 216, 476–483 (2016)
Grant, D.J.W., Mehdizadeh, M., Chow, A.H.-L., Fairbrother, J.E.: Non-linear van't Hoff solubility-temperature plots and their pharmaceutical interpretation. Int. J. Pharmaceutics 18(1–2), 25–38 (1984)
Acree, W.E.: Mathematical representation of thermodynamic properties: Part 2. Derivation of the combined nearly ideal binary solvent (NIBS)/Redlich–Kister mathematical representation from a two-body and three-body interactional mixing model. Thermochim. Acta 198(1), 71–79 (1992)
Jouyban, A.: Review of the cosolvency models for predicting solubility of drugs in water–cosolvent mixtures. J. Pharmacy Pharmaceut. Sci. 11(1), 32–58 (2008)
Jouyban, A., Fakhree, M.A.A., Acree, W.E.: Comment on “Measurement and correlation of solubilities of (Z)-2-(2-aminothiazol-4-yl)-2-methoxyiminoacetic acid in different pure solvents and binary mixtures of water + (ethanol, methanol, or glycol)”. J. Chem. Eng. Data 57(4), 1344–1346 (2012)
Vahdati, S., Shayanfar, A., Hanaee, J., Martínez, F., Acree, W.E., Jouyban, A.: Solubility of carvedilol in ethanol + propylene glycol mixtures at various temperatures. Ind. Eng. Chem. Res. 52(47), 16630–16636 (2013)
Apelblat, A., Manzurola, E.: Solubilities of o-acetylsalicylic, 4-aminosalicylic, 3,5-dinitrosalicylic, and p-toluic acid, and magnesium-DL-aspartate in water from T = (278 to 348) K. J. Chem. Thermodyn. 31(1), 85–91 (1999)
Acree, W.E., Zvaigzne, A.I.: Thermodynamic properties of non-electrolyte solutions: Part 4. Estimation and mathematical representation of solute activity coefficients and solubilities in binary solvents using the NIBS and Modified Wilson equations. Thermochim. Acta. 178, 151–167 (1991)
Meng, Z., Hu, Y., Kai, Y., Yang, W., Cao, Z., Shen, F.: Thermodynamics of solubility of thiomalic acid in different organic solvents from 278.15 K to 333.15 K. Fluid Phase Equil. 352, 1–6 (2013)
Noubigh, A., Jeribi, C., Mgaidi, A., Abderrabba, M.: Solubility of gallic acid in liquid mixtures of (ethanol plus water) from (293.15 to 318.15) K. J. Chem. Thermodyn. 55, 75–78 (2012)
Xie, Y., Shi, H., Du, C., Cong, Y., Wang, J., Zhao, H.: Thermodynamic models for determination of 3-chloro-N-phenylphthalimide solubility in binary solvent mixtures of (acetone, ethyl acetate or 1,4-dioxane + methanol). J. Chem. Thermodyn. 100, 22–28 (2016)
Moodley, K., Rarey, J., Ramjugernath, D.: Experimental solubility for betulin and estrone in various solvents within the temperature range T = (293.2 to 328.2) K. J. Chem. Thermodyn. 98, 42–50 (2016)
Ha, E.-S., Kim, J.-S., Kuk, D.-H., Ha, D.-H., Baek, I.-H., Kim, M.-S.: Determination and correlation of solubility of pranlukast hemihydrate in five organic solvents at different temperatures and its dissolution properties. J. Mol. Liquids 225, 231–234 (2017)
Li, X., Han, S., Zhao, H.: Solubility measurement and modelling of ethyl 5-amino-4-cyano-3-(2-ethoxy-2-oxoethyl)-2-thiophenecarboxylate in four groups mixed solvents. J. Chem. Thermodyn. 103, 414–422 (2016)
Li, R., Han, S., Du, C., Cong, Y., Wang, J., Zhao, H.: Solubility measurement and correlation of 4-nitrophthalimide in (methanol, ethanol, or acetone) + N, N-dimethylformamide mixed solvents at temperatures from 273.15 K to 323.15 K. J. Chem. Thermodyn. 103, 99–106 (2016)
Cao, Z., Hu, Y., Li, J., Liang, M., Liu, Y., Yang, W.: Measurement and correlation solubility of sodium succinate in binary mixed solvents of water + (methanol or ethanol) mixtures. J. Solution Chem. 43(5), 821–835 (2014)
Chen, J., Chen, G., Cong, Y., Du, C., Zhao, H.: Solubility of 2-isopropylimidazole in nine pure organic solvents and liquid mixture of (methanol + ethyl acetate) from T = (278.15 to 313.15) K: Experimental measurement and thermodynamic modelling. J. Chem. Thermodyn. 107, 133–140 (2017)
Li, W., Wang, J., Yao, G., Zhao, H.: Solubility and dissolution thermodynamic properties of 3,3',4,4'-oxydiphthalic anhydride in binary aqueous solutions of acetic acid and propionic acid from (278.15 to 313.15) K. J. Solution Chem. 44(10), 2042–2060 (2015)
Jiang, S., Qin, Y., Wu, S., Xu, S., Li, K., Yang, P., Zhao, K., Lin, L., Gong, J.: Solubility correlation and thermodynamic analysis of sorafenib free base and sorafenib tosylate in monosolvents and binary solvent mixtures. J. Chem. Engi. Data 62(1), 259–267 (2017)
Chen, X., Zhou, Z., Chen, J., Chu, C., Zheng, J., Wang, S., Jia, W., Zhao, J., Li, R., Han, D.: Solubility determination and thermodynamic modeling of buprofezin in different solvents and mixing properties of solutions. J. Chem. Eng. Data 64(3), 1177–1186 (2019)
Li, W.X., Wang, J., Yao, G.B., Zhao, H.K.: Solubility and dissolution thermodynamic properties of 3,3',4,4'-oxydiphthalic anhydride in binary aqueous solutions of acetic acid and propionic acid from (278.15 to 313.15) K. J. Solution Chem. 44(10), 2042–2060 (2015)
Zhou, X., Fan, J., Li, N., Du, Z., Ying, H., Wu, J., Xiong, J., Bai, J.: Solubility of L-phenylalanine in water and different binary mixtures from 288.15 to 318.15 K. Fluid Phase Equil. 316, 26–33 (2012)
Shu, M., Zhu, L., Yuan, M., Wang, L., Wang, Y., Yang, L., Sha, Z., Zeng, M.: Solubility and solution thermodynamic properties of 4-(4-hydroxyphenyl)-2-butanone (raspberry ketone) in different pure solvents. J. Solution Chem. 46(11), 1995–2013 (2017)
Acknowledgements
The authors are grateful for the financial support from the Major National Science and Technology Projects 2017ZX09101001, National Key Research and Development Projects 2017YFD0201406, Major National Scientific Instrument Development Projects of China 21527812, and National Natural Science Foundation of China 21706183.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, B., Wu, D., Zhu, H. et al. Solubility Measurements and Thermodynamic Properties of Sorbic Acid in Binary Solvent Mixtures of (Ethanol, 1-Propanol or 2-Propanol + Water) from 283.15 to 323.15 K. J Solution Chem 49, 1068–1081 (2020). https://doi.org/10.1007/s10953-020-01011-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-020-01011-0