Abstract
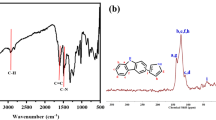
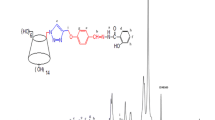
By reacting cyclodextrin-grafted thiacalix [4]arene derivative 9 containing three alkynyl groups with bis-azido compound 6, the first example of cyclodextrin-grafted thiacalix [4]arene netty polymer 10 was designed and prepared by the click chemistry in yield of 76 %. Its structure was confirmed by elemental analysis, 1H NMR spectrum, FTIR spectrum and XRD curve. The M n of polymer 10 was 48,677, indicating average approximately 22 units of compound 9 in each polymer molecule. Its SEM image showed porous and loose morphology. Adsorption experiments of polymer 10 indicated that it possessed excellent adsorption capacities for both cationic and anionic dyes [Orange G sodium salt (OG), Brilliant ponceau 5R (BP), Victoria blue B (VB), Crystal violet (CV), Neutral red (NR) and Methylene blue (MB)]. The highest adsorption capacities for VB, CV, NR and MB were 5.821, 6.825, 6.135 and 5.381 mmol/g, respectively. Adsorption kinetics and isotherms analysis suggested that all the adsorption processes were exothermic and spontaneous. The adsorption processes obeyed pseudo second-order model and the Langmuir isotherm equation. The adsorption abilities of polymer 10 at pH values 3–11 were keep stable between 1.5–4 mmol/g. The adsorption abilities for anionic dyes decreased with the increase of pH values but opposite phenomena for cationic dyes. Polymer 10 showed good reused adsorption property after desorption in five times’ cycles.












Similar content being viewed by others
References
Wong Y, Yu J (1991) Laccase-catalyzed decolorization of synthetic dyes. Water Res 33:3512–3520
Bumpus J (1995) Microbial degradation of health risk compounds. In: Singh VP (ed) Inbiotransformations: Microbial degradation of azo dyes. Elsevier Science BV, Amsterdam, pp. 157–176
Peternel IT, Koprivanac N, Bozic AML, Kusic HM (2007) Comparative study of UV/TiO2, UV/ZnO and photo-Fenton processes for the organic reactive dye degradation in aqueous solution. J Hazard Mater 148:477–484
Crini G, Badot PM (2008) Application of chitosan, a natural aminopolysaccharide, for dye removal from aqueous solutions by adsorption processes using batch studies: a review of recent literature. Prog Polym Sci 33:399–447
Dhodapkar R, Pande NNR, Nandy T, Devotta S (2007) Adsorption of cationic dyes on jalshakti (R), super absorbent polymer and photocatalytic regeneration of the adsorbent. React Funct Polym 67:540–548
Haider S, Binagag F, Haider A, Al-Masry WA (2014) Electrospun oxime-grafted-polyacrylonitrile nanofiber membrane and its application to the adsorption of dyes. J Polym Res 21:371–384
Ansari R, Keivani MB, Ansari R (2011) Application of polyaniline nanolayer composite for removal of tartrazine dye from aqueous solutions. J Polym Res 18:1931–1939
Dabrowski A (2001) Adsorption - from theory to practice. Adv Colloid Interf Sci 93:135–224
Akceylan E, Bahadir M, Yilmaz M (2009) Removal efficiency of a calix[4]arene-based polymer for water-soluble carcinogenic direct azo dyes and aromatic amines. J Hazard Mater 162:960–966
Yilmaz A, Yilmaz E, Yilmaz M (2007) Removal of azo dyes from aqueous solutions using calix[4]arene and cyclodextrin. Dyes Pigments 74:54–60
Kamboh MA, Solangi IB, Memon S (2011) Synthesis and application of p-tert-butylcalix[8]arene immobilized material for the removal of azo dyes. J Hazard Mater 186:651–656
Chen M, Shang T, Fang W, Diao G (2011) Study on adsorption and desorption properties of the starch grafted p-tert-butyl-calix[n]arene for butyl rhodamine B solution. Hazard Mater 185:914–920
Yang FF, Liu WW, Xie JW, Bai XY, Guo HY (2013) Novel deep-cavity calix[4]arene derivatives with s-triazine conjugate systems: synthesis and complexation for dyes. J Incl Phenom Macrocycl Chem 76:311–316
Yang FF, Zhang YM, Guo HY, Wei XL (2013) High efficient liquid membrane transport of dyes using calix[4]arene-linked triphenylene dimers as carriers. Sep Sci Technol 48:1565–1571
Bai XY, Yang FF, Xie JW, Guo HY (2013) Novel 1,2-3,4-bridged and 1,3-bridged calix[4]arene based on large s-triazine conjugate systems: synthesis and complexation for dyes. J Macromol Sci A 50:334–339
Shaikh M, Swamy YM, Pal H (2013) Supramolecular host–guest interaction of acridine dye with cyclodextrin macrocycles: photophysical, pKa shift and quenching study. J Photoch Photobio A 285:41–50
Liu Y, Kang SZ, Zhang HY (2001) Synthesis of cyclodextrin derivative bearing a cyclohexylamino moiety and its inclusion complexation with organic dye molecules. Microchem 70:115–121
Arun KT, Jayaram DT, Avirah RR, Ramaiah D (2011) β-cyclodextrin as a photosensitizer carrier: effect on photophysical properties and chemical reactivity of squaraine dyes. J Phys Chem B 115:7122–7128
Liu Y, Chen Y (2006) Cooperative binding and multiple recognition by bridged bis(β-cyclodextrin)s with functional linkers. Accounts Chem Res 39:681–699
Liu Y, Chen Y, Li L, Huang G, You CC, Zhang HY, Wada T, Inoue Y (2001) Cooperative multiple recognition by novel calix[4]arene-tethered β-cyclodextrin and calix[4]arene-bridged bis(β-cyclodextrin). J Org Chem 66:7209–7215
Liu Y, Li B, You CC, Wada T, Inoue Y (2001) Molecular recognition studies on supramolecular systems. 32. Molecular recognition of dyes by organoselenium-bridged bis(β-cyclodextrin)s. J Org Chem 66:225–232
Zhang HC, Hao AY, Shen J (2008) Progress in cyclodextrin-calix[n]arene and prospect of its future development. Chin J Org Chem 28:954–963
Souchon V, Maisonneuve S, David O, Leray I, Xie J, Valeur B (2008) Photophysics of cyclic multichromophoric systems based on β-cyclodextrin and calix[4]arene with appended pyridin-2′-yl-1,2,3-triazole groups. Photoch Photobio Sci 7:1323–1331
Baruah U, Gogoi N, Majumdar G, Chowdhury D (2015) β-cyclodextrin and calix[4]arene-25,26,27,28-tetrol capped carbondots for selective and sensitive detection of fluoride. Carbohyd Polym 117:377–383
Mulder A, Auletta T, Sartori A, Ciotto SD, Casnati A, Ungaro R, Huskens J, Reinhoudt DN (2004) Divalent binding of a bis(adamantyl)-functionalized calix[4]arene to β-cyclodextrin-based hosts: an experimental and theoretical study on multivalent binding in solution and at self-assembled monolayers. J Am Chem Soc 126:6627–6636
Krause-Heuer AM, Wheate NJ, Tilby MJ, Pearson DG, Ottley CJ, Aldrich-Wright JR (2008) Substituted β-cyclodextrin and calix[4]arene as encapsulatory vehicles for platinum(II)-based DNA intercalators. Inorg Chem 47:6880–6888
Kamboh MA, Solangi IB, Sherazi STH (2011) A highly efficient calix[4]arene based resin for the removal of azo dyes. Desalination 268:83–89
Guo HY, Yang FF, Chai XF, Jiao ZY, Li CC (2012) Synthesis of novel calix[6]-1,4-crown-based netty polymer and its excellent adsorption capabilities for aniline derivatives. Iran Polym J 21:451–456
Yang FF, Huang ZS, Zhang XY, Guo HY (2010) Thiacalix[4]amido-based netty polymers: novel sorbents for heavy metal cations and derivatives of aniline. Iran Polym J 19:309–318
Guo HY, Yang FF, Zhang YM, Di XD (2015) Facile synthesis of mono- and polytopic β-cyclodextrin aromatic aldehydes by click chemistry. Synth Commun 3:338–347
Guo HY, Yang FF, Jiao ZY, Lin JR (2013) Click synthesis and dyes extraction capabilities of novel thiacalix[4]arene derivatives with triazole groups and hydrogen bond groups. Chin Chem Lett 24:450–452
Zhan JY, Tian DM, Li HB (2009) Synthesis of calix[4]crowns containing soft and hard ion binding sites via click chemistry. New J Chem 33:725–728
Chen AH, Chen SM (2009) Biosorption of azo dyes from aqueous solution by glutaraldehyde-crosslinked chitosans. J Hazard Mater 172:1111–1121
Shaker MA, Yakout AA (2016) Optimization, isotherm, kinetic and thermodynamic studies of Pb(II) ions adsorption onto N-maleated chitosan-immobilized TiO2 nanoparticles from aqueous media. Spectrochim Acta A Mol Biomol Spectrosc 154:145–156
Yuan YC, Zhang MQ, Rong MZ (2005) Study on the adsorption behavior of crosslinked chitosan for Ni(II). Acta Chimica Sinca 63:1753–1759
Wang ZH, Xiang B, Cheng RM, Li YJ (2010) Behaviors and mechanism of acid dyes sorption onto diethylenetriamine-modified native and enzymatic hydrolysis starch. J Hazard Mater 180:224–232
Chiou MS, Li HY (2002) Equilibrium and kinetic modeling of adsorption of reactive dye on cross-linked chitosan beads. J Hazard Mater 93:233–248
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Hazard Mater 40:1361–1403
Giles CH, Smith D, Huitson A (1974) General treatment and classification of solute adsorption-isotherm. 1. Theoretical. J Colloid Interf Sci 47:755–765
Chiou MS, Ho PY, Li HY (2004) Adsorption of anionic dyes in acid solutions using chemically cross-linked chitosan beads. Dyes Pigments 60:69–84
Chiou MS, Chuang GS (2006) Competitive adsorption of dye in acid solutions on chemically metanil yellow and RB15 cross-linked chitosan beads. Chemosphere 62:731–740
Elwakeel KZ (2009) Removal of reactive black 5 from aqueous solutions using magnetic chitosan resins. J Hazard Mater 167:383–392
Yang FF, Bai XY, Xu BT, Guo HY (2013) Triphenylene-modified chitosan: novel high efficient sorbent for cationic and anionic dyes. Cellulose 20:895–906
Yoshida H, Okamoto A, Kataoka T (1993) Adsorption of acid dye on cross-linked chitosan fibers – equilibria. Chem Eng J 48:2267–2272
Acknowledgments
Financial support from the National Natural Science Foundation of China (No: 21406036), Fujian Natural Science Foundation of China (No. 2014 J01034), Key projects of science and technology of Fujian province (2014 N0025) and the Program for Innovative Research Team in Science and Technology in Fujian Province University were greatly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, S., Guo, H., Yang, F. et al. Cyclodextrin-grafted thiacalix [4]arene netty polymer based on the click chemistry: preparation and efficient adsorption for organic dyes. J Polym Res 23, 28 (2016). https://doi.org/10.1007/s10965-016-0920-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-016-0920-x