Abstract
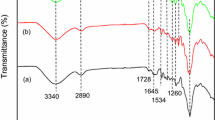
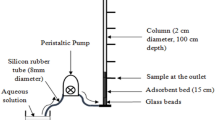
This study described adsorption of uranium(VI) by citric acid modified pine sawdust (CAMPS) in batch and fixed-bed column modes at 295 K. The equilibrium adsorption data were analyzed by Langmuir, Freundlich, Koble–Corrigan and Dubinin–Radushkevich isotherm models. The results indicated that the Langmuir and Koble–Corrigan models provided the best correlation of the experimental data. The Elovish model was better to fit the kinetic process, which suggested that ion exchange was one of main mechanism. The effective diffusion parameter D i values indicated that the intraparticle diffusion was not the rate-controlling step. In fixed-bed column adsorption, the effects of bed height, feed flow rate, and inlet uranium (VI) concentration were studied by assessing breakthrough curve. The Thomas, the Yan and the bed-depth/service time (BDST) models were applied to the column experimental data to determine the characteristic parameters of the column adsorption. The results were implied that CAMPS may be suitable as an adsorbent material for adsorption of uranium (VI) from an aqueous solution.








Similar content being viewed by others
References
Donat R, Esen K, Cetisli H, Aytas S (2009) J Radioanal Nucl Chem 279:253
Humelnicu D, Drochioiu G, Sturza MI, Cecal A, Popa K (2006) J Radioanal Nucl Chem 270:637
Donia AM, Atia AA, Moussa MM, Sherif AM, Magied MO (2009) Hydrometallurgy 95:183
Ganesh R, Robinson KG, Chu LL, Kucsmas D, Reed GD (1999) Water Res 33:3447
Kryvoruchko AP, Yurlova LY, Atamanenko ID, Kornilovich BY (2004) Desalination 162:229
Mellah A, Chegrouche S, Barkat M (2006) J Colloid Interface Sci 296:434
Kadous A, Didi M, Villemin D (2009) J Radioanal Nucl Chem 280:157
Sodayea H, Nisanb S, Poletikoc C, Prabhakara S, Tewaria PK (2009) Desalination 235:9
Morsy AMA, Hussein AEM (2011) J Radioanal Nucl Chem 288:341
Zou WH, Zhao L, Han RP (2011) J Radioanal Nucl Chem 288:239
Zou WH, Bai HJ, Zhao L, Li K, Han RP (2011) J Radioanal Nucl Chem 288:779
Aytas SO, Akyil S, Eral M (2004) J Radioanal Nucl Chem 260:119
Bishay AF (2010) J Radioanal Nucl Chem 286:81
Bagherifam S, Lakzian A, Ahmedi SJ, Rahimi MF, Halajnia A (2010) J Radioanal Nucl Chem 283:289
Vaughan T, Seo CW, Marshall WE (2001) Bioresour Technol 78:133
Šćiban M, Klašnja M, Škrbić B (2008) Desalination 229:170
O’Connell DW, Birkinshaw C, O’Dwyer TF (2008) Bioresour Technol 99:6709
Sciban M, Radetic B, Kevresan Z, Klasnja M (2007) Bioresour Technol 98:402
Zhang H, Tang Y, Liu XN, Ke ZG, Su X, Cai DQ, Wang XQ, Liu YD, Huang Q, Yu ZL (2011) Desalination 274:97
Misaelides P, Godelitsas A, Filippidis A, Charistos D, Anousi I (1995) Sci Total Environ 173/174:237
Guibal E, Lorenzelli R, Vincent T, Cloirec PL (1995) Environ Technol 16:101
Balistrieri LS, Murray JW (1981) Am J Sci 281:788
Barnett MO, Jardine PM, Brooks SC, Selim HM (2000) Soil Sci Soc Am J 64:908
Langmuir I (1916) J Am Chem Soc 38:2221
Freundlich HMF (1906) J Phys Chem 57:385–470
Koble RA, Corrigan TE (1952) Ind Eng Chem 44:383
Dubinin MM (1960) Chem Rev 60:235
Hasany SM, Chaudhary MH (1996) Appl Radiat Isot 47:467
Mahramanlioglu M, Bicer IO, Misirli T, Kilislioglu A (2007) J Radioanal Nucl Chem 273:621
Chowdhury S, Mishra R, Saha P, Kushwaha P (2011) Desalination 265:159
Ho YS, Ng JCY, McKay G (2000) Sep Purif Methods 29:189
Cheung CW, Porter JF, Mckay G (2000) Sep Purifi Technol 19:55
Weber WJ Jr, Morris JC (1963) Am Soc Civil Eng 89:31
Srivastava VC, Swamy MM, Mall ID, Prasad B, Mishra IM (2006) Colloid Surf A 272:89
Dogan M, Ozdemir Y, Alkan M (2007) Dyes Pig 75:701
Singh KK, Rastogi R, Hasan SH (2005) J Colloid Interface Sci 290:61
Aksu Z, Gonen F (2004) Process Biochem 39:599
Gokhale SV, Jyoti KK, Lele SS (2009) J Hazard Mater 170:735
Vijayaraghavan K, Jegan J, Palanivelu K, Velan M (2004) J Hazard Mater 113:223
Thomas HC (1944) J Am Chem Soc 66:1664
Vijayaraghavan K, Prabu D (2006) J Hazard Mater 137:558
Yan G, Viraraghavan T, Chen M (2001) Adsorpt Sci Technol 19:25
Lodeiro P, Herrero R, Sastre de Vicente ME (2006) J Hazard Mater 137:244
Goel J, Kadirvelu K, Rajagopal C, Garg VK (2005) J Hazard Mater 125:211
Acknowlegments
This study was supported by the Education Department of Henan Province in China (No. 2010A610003) and Henan Science and Technology Department in China (No. 102102210103).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zou, W., Zhao, L. Removal of uranium(VI) from aqueous solution using citric acid modified pine sawdust: batch and column studies. J Radioanal Nucl Chem 292, 585–595 (2012). https://doi.org/10.1007/s10967-011-1452-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-011-1452-9