Abstract
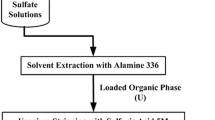
This paper describes the solvent extraction studies carried out on an acidic low assay uranium bearing leach liquor generated during sulfuric acid leaching of a refractory uranium ore using alamine 336–isodecenol–kerosene reagent combine. The leach liquor has a U3O8 content of about 270 mg/L, free acidity 2.4 N H2SO4 and total dissolved solids concentration of 260 g/L. Process parameteric variation studies indicated strong influence of free acidity of the leach liquor, alamine 336 concentration and aqueous to organic phase ratio on the extraction efficiency of uranium. An extraction efficiency of about 95% was achieved when the free acidity of leach liquor was 1 N H2SO4 or lower, using 2% (v/v) alamine 336 at ambient temperature with an aqueous to organic phase ratio of 1:1. The loading capacity under these conditions was 1.2 g/L of U3O8. About 98% of the uranium values could be stripped from the loaded organic using 1 N NaCl in 0.2 N H2SO4. The solvent extraction studies aided in developing a suitable process flowsheet for treating refractory uranium ores which need high acidity during leaching and relatively lower acidity for purification by solvent extraction.





Similar content being viewed by others
References
Merrit R. C. (1971) The extractive metallurgy of uranium. Colorado School of Mines Research Institute, Colorado, pp 61–62
Edwards CR, Oliver JA (2000) Uranium processing: a review of currents methods and technologies. J Met 52(9):12–20
Anon (1993) Uranium extraction technology. Technical Reports Series No. 359, International Atomic Energy Agency, Vienna, December, p 355
Anon (1980) Significance of mineralogy in the development of flowsheet for processing uranium ores. Technical Report Series No. 196, International Atomic Energy Agency, Vienna
Ritcey GM (2006) Solvent extraction in hydrometallurgy: present and future. Tsinghua Sci Technol 11:137–152
Lunt D, Boshoff P, Boylett M, El-Ansary Z (2007) Uranium extraction: the key process drivers. J Southern Afr Inst Min Metall 107:419–426
Paatero E, Nyman B, Hakkarainen J (2010) Recovery of molybdenum and uranium as by-products with solvent extraction, metals and materials recycling and recovery. 1st International workshop, April 2010, Santiago, Chile
van Tonder D, Kotze M (2007) Uranium recovery from acid leach liquors: IX or SX. In: ALTA 2007. Uranium Conference, Perth, Western Australia. www.altamet.com.au
Mackenzie JMW (1997) Uranium solvent extraction using tertiary amines. Uranium Ore Yellow Cake Seminar, February 1997, Melbourne, Australia
Morais CA, Gomiero LA (2005) Uranium stripping from tertiary amine loaded solution by ammonium sulfate. Miner Eng 18:1277
Bouhonl Ali M, Badjah Hadj Ahmed AY, Attou M, Elias AH, Didi MA (2010) The solvent extraction of uranium (VI) using hydroxyalkylenediphosphonic acids. J Appl Sci 10(22):2905–2910
Kumar JR, Kim J-S, Lee J-Y, Yoon H-S (2010) Solvent extraction of uranium(VI) and separation of vanadium(V) from sulfate solutions using Alamine 336. J Radioanal Nucl Chem 285:301–308
Collet S, Chagnes A, Courtaud B, Thiry J, Cotea G (2009) Solvent extraction of uranium from acidic sulfate media by alamine 336: computer simulation and optimization of the flow-sheets. J Chem Technol Biotechnol 84:1331
Yu C, Guoxin S, Zhenwei Z, Yufen H, Sixiu S (2007) Extraction of U (VI) with N, N′-dimethyl-N, N′-dioctylsuccinylamide in toluene. J Radioanal Nucl Chem 272:199–201
Lapka JL, Paulenova A, Alyapyshev MY, Babain VA, Herbst RS, Law JD (2009) Extraction of uranium (VI) with diamides of dipicolinic acid from nitric acid solutions. Radiochim Acta 97:291–296
El-Reefy SA, Awwad NS, Aly HF (1997) Liquid–liquid extraction of uranium from phosphoric acid by HDEHP-Cyanex 921 mixture. J Chem Technol Biotechnol 69:271–275
Daoud JA, Zeid MM, Aly HF (1997) Tetravalent uranium extraction by HDEHP in kerosene from phosphate medium. Sol Extr Ion Exchange 15:203–217
Singh DK, Singh H, Mathur JN (2001) Synergistic extraction of U(VI) with mixtures of 2 ethyl hexyl phosphonic acid-mono-2 ethyl hexyl ester (PC 88A) and TBP, TOPO. Radiochim Acta 89:573–578
Mathur JN, Ruikar PB, Krishna MVB, Murali MS, Nagar MS, Iyer RH (1996) Extraction of Np(IV), Np(VI), Pu(IV) and U(VI) with amides, BEHSO and CMPO from nitric acid medium. Radiochim Acta 73:199–206
Khan MH, Shahida S, Ali A (2008) Liquid–liquid extraction of uranium from nitric acid solution using di-n-butylsulfoxide in petroleum ether as extractant. Radiochim Acta 96:35–40
Acknowledgments
The authors are thankful Dr. A.K.Suri, Director, Materials Group, BARC for his interest in the work and encouragement. The authors thank the reviewers for their critical comments and helping in improving the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramadevi, G., Sreenivas, T., Navale, A.S. et al. Solvent extraction of uranium from lean grade acidic sulfate leach liquor with alamine 336 reagent. J Radioanal Nucl Chem 294, 13–18 (2012). https://doi.org/10.1007/s10967-011-1507-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-011-1507-y