Abstract
The uranium(VI) accumulation was studied in detail by using the biomass of mangrove endophytic fungus Fusarium sp.#ZZF51 from the South China Sea. The uranium(VI) biosorption process onto the tested fungus powders was optimized at pH 4.0, adsorption time 60 min, and uranium(VI) initial concentration 50 mg L−1 with 61.89% of removal efficiency. According to Fourier transform infrared spectra for the tested fungus before and after loaded with uranium(VI), the results showed that both of hydroxyl and carboxyl groups acted as the important roles in the adsorption process. In addition, the experimental data were analyzed by using parameter and kinetic models, and it was obtained that the Langmuir isotherm model and the pseudo-second-order kinetic model provided better correlation with the experimental data for adsorption of uranium(VI).
Similar content being viewed by others
Introduction
Uranium is not only a main raw material for nuclear industry, but also a toxic radioactivity element. For this reason, the recovery, accumulation, and removal of uranium are of great importance [1]. Uranium exists normally in the environment as aqueous uranyl ion (UO2 2+) [2]. At present, recovery of uranium(VI) from dilute aqueous solution commonly includes coagulation, chromatographic extraction, chemical precipitation, ion exchange, membrane dialysis, etc. [3, 4], but they have several disadvantages, like clogging, high cost and ineffectiveness when uranium(VI) ions are present in the wastewater at low concentrations, especially in the range of 1–100 mg L−1 [5, 6]. Therefore, the above methods have limitation in application.
Biosorption is a process that the water-solubility metal ions are absorbed by certain organisms’ chemical structures and then removed by solid–liquid separation [7–9]. To accumulate uranium(VI) by micro-organisms from the inland has been widely studied for the past decades [10, 11], while microorganisms from ocean including mangrove endophytic source are rarely reported. It is well known that marine environment is of many special natures such as high salt, high pressure, low temperature, low light, poor nutrition, and so on, which result marine microorganisms have theirs own special species and metabolic approaches, and produced a lot of novel metabolites [12, 13], so research on the biosorption of uranium(VI) by using marine microorganisms will be of greater importance.
Mangrove endophytic fungus Fusarium sp. #ZZF51 was collected from Chinese Zhanjiang sea area. The initial work showed that it had strong ability of absorption copper(II), which was not relying on a simple penetration of passive absorption, but on the “pump” type of active biological adsorption [14]. In order to systematically and comprehensively research the biosorption of heavy metals by the tested fungus, this paper studied the uranium(VI) adsorption process. Many influencing factors about uranium(VI) adsorption such as contact time, solution pH and initial uranium(VI) concentration were discussed, and the suitable adsorption isotherm and kinetic models were also determined.
Materials and methods
Materials
A stock solution of 500 mg L−1 of uranium(VI) was prepared by dissolving uranyl nitrate in deionized water and further diluted to the concentrations required for the experiments. The initial pH of each working solution was adjusted by HCl and NaOH solutions at the start of the experiment. 0.1% Arsenazo-III solution was prepared by dissolving 0.5 g of the reagent in 500 mL of deionized water. All of those used reagents were of analytical-reagent grade.
Preparation of the biosorption materials
Fusarium sp. #ZZF51 was obtained from the South China Sea coast (Zhanjiang sea area) and deposited with Department of Applied Chemistry and School of Life Sciences, Sun Yat-sen (Zhongshan) University, Guangzhou, China.
Culture conditions
Starter cultures were transferred to a 250 mL Erlenmeyer flask containing 150 mL of liquid medium GPY (glucose 10 g L−1, peptone 2 g L−1, yeast extract 1 g L−1, sea salt 2 g L−1). The flask was incubated at 30°C on a rotary shaker for 5–7 days, and the mycelium was aseptically transferred to a 500 mL Erlenmeyer flask containing liquid medium (300 mL). The flask was then incubated at room temperature for 22 days. The mycelium was, respectively filtered, collected, dried, and grinded, and the 100 mesh powders were obtained.
Batch adsorption experiments
A series of batch adsorption experiments were carried out in 250 mL Erlenmeyer flasks containing 50 mL of uranium(VI) solution, the initial pH of solution was adjusted to desired values by HCl or NaOH solution. 0.1 g tested fungus powders were thrown into 50 mL uranium(VI) solution, then the flasks were transferred into the thermostat oscillator shaker for 60 min by controlling the room temperature. At the end of the adsorption, the aqueous samples (2 mL) were taken and centrifuged for 10 min at 3,000 rpm, and the concentrations were analyzed using a 721-spectrophotometer. All the experiments were carried out independently in triplicate and repeated twice at least. The adsorption amount (Q e) and the removal percentage (η%) were calculated according to Eqs. 1 and 2:
where Q e (mg g−1) is the equilibrium biosorption capacity, C 0 and C e are the initial and equilibrium solution concentrations(mg L−1), V is the volume of the solution (L), W is the weight of fungus powers used (g) and η is the removal efficiency.
Fourier transforms infrared (FTIR) spectra experiments
Fourier transforms infrared (FTIR) spectra were used to probe the functional groups on the surface of the fungus Fusarium sp. #ZZF51. The spectra were recorded with a spectrometer within the range 400–4,000 cm−1 using a KBr window. The KBr background was automatically subtracted from the sample spectra.
Results and discussion
Fourier transforms infrared (FTIR) spectra characterization
The FTIR spectra of the virgin and uranium(VI) loaded biosorbents are respectively shown in Fig. 1a and b. As seen in Fig. 1a, a characteristic strong and broad band appears at 3,408 cm−1, corresponding to the stretching vibration of –NH2 group and –OH group. The peak at 1,550 cm−1 attributes to the N–H in-plane bending vibration of secondary amide CONH (amide II band). The shoulder band at about 1,649 cm−1 belongs to C=O stretching vibration. The absorption bands at 2,920 and 2,850 cm−1 are assigned to the asymmetric and symmetric C–H stretching of the aliphatic groups, respectively. The region 1,300–1,000 cm−1 contains absorption bands of phosphate groups.
Compared with FTIR spectra of virgin roots, after biosorption of uranium(VI) for 60 min, the peak at 1,550 cm−1 disappears in this spectrum. The FTIR spectroscopic analysis of uranium(VI)-loaded biosorbent of fungus Fusarium sp.#ZZF51 indicated intensity shifted strong asymmetrical stretching bands at 3,452 cm−1 (indicative of –OH and –NH groups) and 1,631 cm−1 (indicative of C=O groups) when compared with that of unloaded biomass which showed the same absorption at 3,408 and 1,649 cm−1, respectively. These observations indicated the involvement of these functional groups in the biosorption process [15–17].
Effect of initial solution pH
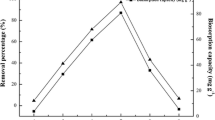
The biosorption capacity of fungus Fusarium sp.#ZZF51 for uranium(VI) was strongly affected by the initial pH value of aqueous solution. The experiment was investigated by using 50 mL of uranium(VI) 50 mg L−1 with pH range of 2.0 to 7.0 at room temperature for 60 min. The influence of the initial pH on uranium(VI) adsorption was given in Fig. 2.
As seen in Fig. 2, it is clear that the biosorption capacity of fungus Fusarium sp.#ZZF51 for uranium(VI) was strongly affected by the initial pH value of aqueous solution. The uranium(VI) biosorption capacity and the percentage of adsorption increases with increasing pH to a maximum value (when pH is 4.0) and then declines rather rapidly with further increase of pH. The influence of pH on uranium(VI) adsorption can be explained in the following way: In strong acidic solutions (pH < 4.0), more protons occupy a lot of the active adsorption sites, as reduces the number of binding sites for the adsorption of UO2 2+, therefore, the removal efficiency of uranium(VI) is lower in strong acidic solutions (pH < 4.0). However, when pH value increases beyond 4.0, a large number of active adsorption sites are released, and the availability of free uranium(VI) ions is maximum at pH 4.0 and hence maximum adsorption. When pH value is over 4.0, uranium(VI) ions are hydrolyzed, hydrolysis products include UO2(OH)+ (UO2)2(OH) 2+2 , (UO2)3(OH) +5 , which results in the decline of biosorption capacity and adsorption removal efficiency of uranium(VI) [18, 19].
Effect of contact time on the adsorption
The effect of contact time on the adsorption removal efficiency and adsorption capacity was studied at a fixed mass of fungus Fusarium sp.#ZZF51 (0.1 g) and room temperature and initial pH (4.0), and contact times varying from 10 to 180 min. The result is presented in Fig. 3.
It is clear in Fig. 3 that the adsorption capacity and removal efficiency of uranium (VI) by fungus Fusarium sp.#ZZF51 increases with an increase of contact time until adsorption equilibrium within 60 min. The biosorption process was very fast within 60 min and with 61.89% uranium(VI) uptake taking place at 60 min. The reason can be explained as: adsorption of uranium(VI) is found to be a two stage process, consisting of an initial rapid passive binding of metals to negatively charged sites on the cell walls, followed by a slower active uptake of metal ions into the cells. The first phase is interpreted to be the instantaneous adsorption stage or external surface adsorption. The second phase is considered to be the gradual adsorption stage where intraparticle diffusion controls the adsorption rate until finally equilibrium [19, 20].
Effect of initial uranium(VI) concentration
The adsorption experiments were finished under the conditions of 0.1 g adsorbent, pH 4.0, and initial uranium(VI) concentrations varing from 20 to 70 mg L−1. Their findings were plotted in Fig. 4.
As shown in the Fig. 4, the biosorption capacity of uranium(VI) increased with the increasing initial uranium(VI) concentration in the aqueous solution. When the uranium(VI) ions concentrations ranged from 20 to 70 mg L−1, the removal percentage of uranium(VI) at higher concentration levels showed a decreasing trend. The maximum removal efficiency of uranium(VI) (61.89%) was obtained when the initial uranium(VI) concentration was 50 mg L−1. This may be due to the saturation of the adsorption sites for uranium(VI) ions.
Adsorption isotherm
Equilibrium data is a piece of vital information in researching adsorption process. At present, the most popular equations are Langmuir and Freundlich parameter models. The Langmuir model [21] is presented by the following equation:
where Q e and Q m are the equilibrium and maximum uranium(VI) adsorption capacity, C e is the equilibrium solution concentrations, and K a is the equilibrium constant. The Langmuir isotherm plot is shown in Fig. 5.
The Freundlich model proposes a monolayer sorption with a heterogeneous energetic distribution of active sites, and with interaction of sorbed molecules, Freundlich model [1, 2] is given as:
This expression can be linearized and given:
where C e is the equilibrium uranium(VI) concentration (mg L−1), Q e is the amount of metal biosorbed on the biosorbent (mg g−1), Kb and n are the Freundlich constants which feature the system, respectively. A plot of ln Q e versus ln C e would result in a straight line with intercept of ln K b as seen in Fig. 6.
In order to assess the different adsorption isotherms and their ability to correlate with experimental results, a comparison of coefficients that were determined for the two adsorption isotherms has been made and listed in Table 1.
It can be seen from Table 1 that both the Langmiur and Freundlich isotherm models fitted with the experimental data well and the former model was a better fit than the latter.
Adsorption kinetics
Pseudo-first-order kinetic model [6] was employed to fit the kinetic data as Eq. 6:
where Q t is the amount of the adsorbed uranium(VI) (mg g−1) at time t (min) and K 1 (1 min−1) is the adsorption rate constant of the pseudo-first-order equation. Parameters of the pseudo-first-order equations were calculated from the intercept and slope of the ln(Q e−Q t) versus t in Fig. 7. The values of pseudo-first-order equation parameters together with correlation coefficients are given in Table 2. The correlation coefficients for the pseudo-first-order equation obtained at all of the studied concentrations were low.
Pseudo-second-order kinetics model [5] was employed as the following formulation:
where K 2 is the adsorption rate constant of pseudo-second-order equation. The pseudo-second-order plots are given in Fig. 8. As seen from Fig. 8, the pseudo-second-order equation provides the better correlation for two of the biosorption process, the values of pseudo-second-order equation parameters together with correlation coefficients are listed in Table 2.
Conclusions
The uranium(VI) adsorption by fungus Fusarium sp. #ZZF51 was dependent on contact time, pH, and initial uranium(VI) concentration. Uranium(VI) biosorption process onto fungus powders was optimized at pH 4.0, adsorption time 60 min and uranium(VI) initial concentration 50 mg L−1 with 61.89% of removal efficiency. By comparing with Fourier transform infrared spectra for the tested fungus before and after loaded with uranium(VI), hydroxyl and carboxyl groups have an important contribution to the adsorption process. The experimental data were analyzed by using parameter and kinetic models, and it was obtained that the Langmuir isotherm model and the pseudo-second-order kinetic model provided better correlation with the experimental data for adsorption of uranium (VI).
References
Mellah A, Chegrouche S, Barkat M (2006) The removal of uranium(VI) from aqueous solutions onto activated carbon: Kinetic and thermodynamic investigations. J Col Inter Sci 296:434–441
Anirudhan TS, Bringle CD, Rijith S (2010) Removal of uranium(VI) from aqueous solutions and nuclear industry effluents using humic acid-immobilized zirconium-pillared clay. J Env Rad 101:267–276
Donia MA, Atia AA, Ewais M (2009) Removal of uranium(VI) from aqueous solutions using glycidyl methacrylate chelating resins. Hydrometallurgy 95:183–189
Kulkarni PS, Mukhopadhyay S, Bellary MP, Ghosh SK (2002) Studies on membrane stability and recovery of uranium (VI) from aqueous solutions using a liquid emulsion membrane process. Hydrometallurgy 64:49–58
Wang JS, Hu XJ, Liu YG, Xie SB, Bao ZL (2010) Biosorption of uranium (VI) by immobilized Aspergillus fumigatus beads. J Env Radio 101:504–508
Sangi MR, Shahmoradi A, Zolgharnein J, Azimi GH, Ghorbandoost M (2008) Removal and recovery of heavy metals from aqueous solution using Ulmus carpinifolia and Fraxinus excelsior tree leaves. J Haza Mater 155:513–522
Wang JL, Chen C (2009) Biosorbents for heavy metals removal and their future. Biotechnol Adv 27:195–226
Babel S, Kurniawan TA (2003) Low-cost adsorbents for heavy metals uptake from contaminated water: a review. J Hazard Mater 97:219–243
Bhat SV, Melo JS, Chaugule BB, D’Souz SF (2008) Biosorption characteristics of uranium(VI) from aqueous medium onto Catenella repens, a red alga. J Hazard Mater 158:628–635
Kalin M, Wheeler WN, Meinrath G (2005) The removal of uranium from mining waste water using algal/microbial biomass. J Environ Radioact 78:151–177
Cui P, Yun HL, Xiao HC, Rong H, Cai XW, Chun QL (2010) Adsorptive removal of uranium from aqueous solution using chitosan-coated attapulgite. J Radioanal Nucl Chem 286:185–193
Tan N, Shao CL, She ZG, Lin YC (2009) Progress on quinone secondary metabolites of marine microorganisms. Chin J Nat Med 7(1):71–80
Bugni TS, Ireland CM (2004) Marine-derived fungi: a chemically and biologically diverse group of microorganisms. Nat Prod Rep 21:143–163
Tan N, Pan JH, Peng GT (2008) A copper coordination compound produced by a marine fungus Fusarium sp. ZZF51 with biosorption of Cu(II) ions. Chin J Chem 26:516–521
Sevda A, Sinem A, Melek M, Gun B, Nevzat KL (2010) Sorption of uranium using silica gel with benzoylthiourea derivatives. J Radioanal Nucl Chem 285:525–529
Kazy SK, D’Souza SF, Sar P (2009) Uranium and thorium sequestration by a Pseudomonas sp.: mechanism and chemical characterization. J Hazard Mater 163:65–72
Akhtar K, Khalid AM, Akhtar MW, Ghauri MA (2009) Removal and recovery of uranium from aqueous solutions by Ca-alginate immobilized Trichoderma harzianumm. Bioresour Technol 100:4551–4558
Elif AB, Melek M, Muruvvet Y (2010) Preconcentration of uranium (VI) and thorium (IV) from aqueous solutions using low-cost abundantly available sorbent. J Radioanal Nucl Chem 283:471–476
Wang GH, Liu JS, Wang XG, Xie ZY, Deng NS (2009) Adsorption of uranium (VI) from aqueous solution onto cross-linked chitosan. J Hazard Mater 168:1053–1058
Kalsoom A, Akhtar MW, Ahmad M (2007) Removal and recovery of uranium from aqueous solutions by Trichoderma harzianum. Water Res 41:1366–1378
Cem G, Sule A (2009) Biosorption of uranium(VI) from aqueous solution using calcium alginate beads. J Hazard Mater 168:369–375
Acknowledgments
Supported by the Natural Science Foundation of China (No.20072058) and the Science and Technology Development Project of Hunan (No.2010FJ3014).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Yang, H.B., Tan, N., WU, F.J. et al. Biosorption of uranium(VI) by a mangrove endophytic fungus Fusarium sp. #ZZF51 from the South China Sea. J Radioanal Nucl Chem 292, 1011–1016 (2012). https://doi.org/10.1007/s10967-011-1552-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-011-1552-6