Abstract
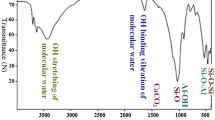
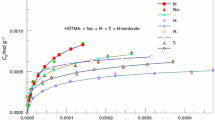
The ability of hexadecyltrimethylammonium cation pillared bentonite (HDTMA+-bentonite) has been explored for the removal and recovery of uranium from aqueous solutions. The adsorbent was characterized using small-angle X-ray diffraction, high resolution transmission electron microscopy, and Fourier transform infrared spectroscopy. The influences of different experimental parameters such as solution pH, initial uranium concentration, contact time, dosage and temperature on adsorption were investigated. The HDTMA+-bentonite exhibited the highest uranium sorption capacity at initial pH of 6.0 and at 80 min. Adsorption kinetics was better described by the pseudo-second-order model and adsorption process could be well defined by the Langmuir isotherm. The thermodynamic parameters, △G° (308 K), ΔH°, and ΔS° were determined to be −31.64, −83.84 kJ/mol, and −169.49 J/mol/K, respectively, which demonstrated the sorption process of HDTMA+-bentonite towards U(VI) was feasible, spontaneous, and exothermic in nature. The adsorption on HDTMA+-bentonite was more favor than Na-bentonite, in addition the saturated monolayer sorption capacity increased from 65.02 to 106.38 mg/g at 298 K after HDTMA+ pillaring. Complete removal (≈100%) of U(VI) from 1.0 L simulated nuclear industry wastewater containing 10.0 mg U(VI) ions was possible with 1.5 g HDTMA+-bentonite.
















Similar content being viewed by others
References
Jackson BP, Ranville JF, Bertsch PM, Sowder AG (2005) Environ Sci Technol 39:2478–2485
Donia AM, Atia AA, Moussa EMM, El-Sherif AM, El-Magied MOA (2009) Hydrometallurgy 95:183–189
Smith SC, Douglas M, Moore DA, Kukkadapu RK, Arey BW (2009) Environ Sci Technol 43:2341–2347
Xie S, Zhang C, Zhou X, Yang J, Zhang X, Wang J (2009) J Environ Radioact 100:162–166
Djedidi Z, Bouda M, Souissi MA, Ben Cheikh R, Mercier G, Tyagi RD, Blais JF (2009) J Hazard Mater 172:1372–1382
Abdel-Khalek AA, Ali MM, Ashour RM, Abdel-Magied AF (2011) J Radioanal Nucl Chem 290:353–359
Kumari N, Prabhu DR, Pathak PN, Kanekar AS, Manchanda VK (2011) J Radioanal Nucl Chem 289:835–843
Cojocaru C, Zakrzewska-Trznadel G, Jaworska A (2009) J Hazard Mater 169:599–609
Cojocaru C, Zakrzewska-Trznadel G, Miskiewicz A (2009) J Hazard Mater 169:610–620
Li X, Song Q, Liu B, Liu C, Wang H, Geng J, Chen Z, Liu N, Li S (2011) Prog Chem 23:1446–1453
Sprynskyy M, Kowalkowski T, Tutu H, Cukrowska EM, Buszewski B (2011) Chem Eng J 171:1185–1193
Hussein AEM (2011) J Radioanal Nucl Chem 289:321–329
Zhao HT, Jaynes WF, Vance GF (1996) Chemosphere 33:2089–2100
Huh JK, Song DI, Jeon YW (2000) Sep Sci Technol 35:243–259
Upson R, Burns S (2006) J Colloid Interface Sci 297:70–76
Hsu YH, Wang MK, Pai CW, Wang YS (2000) Appl Clay Sci 16:147–159
Dentel SK, Jamrah AI, Sparks DL (1998) Water Res 32:3689–3697
Lee JJ, Choi J, Park JW (2002) Chemosphere 49:1309–1315
Oyanedel-Craver VA, Fuller M, Smith JA (2007) J Colloid Interface Sci 309:485–492
Akar ST, Yetimoglu Y, Gedikbey T (2009) Desalination 244:97–108
Majdan M, Pikus S, Gajowiak A, Gładysz-Płaska A, Krzyżanowska H, Żuk J, Bujacka M (2010) Appl Surf Sci 256:5416–5421
Majdan M, Pikus S, Gajowiak A, Sternik D, Zieba E (2010) J Hazard Mater 184:662–670
Liu Y, Cao X, Hua R, Wang Y, Liu Y, Pang C, Wang Y (2010) Hydrometallurgy 104:150–155
Bayramoglu G, Celik G, Arica M (2006) J Hazard Mater 136:345–353
Aytas S, Yurtlu M, Donat R (2009) J Hazard Mater 172:667–674
Hazer O, Kartal Ş (2010) Talanta 82:1974–1979
Parab H, Joshi S, Shenoy N, Verma R, Lali A, Sudersanan M (2005) Bioresour Technol 96:1241–1248
Ghaemi A, Torab-Mostaedi M, Ghannadi-Maragheh M (2011) J Hazard Mater 190:916–921
Psareva T, Zakutevskyy O, Chubar N, Strelko V, Shaposhnikova T, Carvalho J, Correia M (2005) Colloid Surf A 252:231–236
Anirudhan TS, Rijith S, Tharun AR (2010) Colloid Surf A 368:13–22
Anirudhan TS, Divya L, Suchithra PS (2009) J Environ Manag 90:549–560
Donat RJ (2009) Chem Thermodyn 41:829–835
Kilincarslan A, Akyil S (2005) J Radioanal Nucl Chem 264(3):541–548
Zhu W, Liu Z, Chen L, Dong Y (2011) J Radioanal Nucl Chem 289(3):781–788
Zhao D, Yang S, Chen S, Guo Z, Yang X (2011) J Radioanal Nucl Chem 287(2):557–565
Gao L, Yang Z, Shi K, Wang X, Guo Z, Wu W (2010) J Radioanal Nucl Chem 284(3):519–526
Guerra DL, Leidens VL, Viana RR, Airoldi C (2010) J Solid State Chem 183(5):1141–1149
Yusan S, Aslani MAA, Turkozu DA, Aycan HA, Aytas S, Akyil S (2010) J Radioanal Nucl Chem 283(1):231–238
Mellah A, Chegrouche S, Barkat M (2006) J Colloid Interface Sci 296(2):434–441
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Grant No. 21101024), Key Project of Chinese Ministry of Education (Grant No. 211086), Natural Science Foundation of Jiangxi Province (No. 2010GQH0015), Science and Technology project of Jiangxi Provincial Department of Education (No. GJJ11139) and Open Project Foundation of the Key Laboratory of Radioactive Geology and Exploration Technology Fundamental Science for National Defense, East China Institute of Technology, China (2010RGET08).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, YQ., Zhang, Zb., Li, Q. et al. Adsorption of uranium from aqueous solution using HDTMA+-pillared bentonite: isotherm, kinetic and thermodynamic aspects. J Radioanal Nucl Chem 293, 231–239 (2012). https://doi.org/10.1007/s10967-012-1659-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-012-1659-4