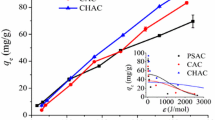
Abstract
Activated carbon can potentially be used as an adsorbent for removing Tc from aqueous solutions. Five carbon materials were prepared by soaking of fibrous cellulose with different solutions containing inorganic compounds suitable for creation of micropores. After drying, materials were carbonized at 500–800 °C, characterized by BET, acid–base titration, HRTEM and SAXRD methods and tested on their adsorption capabilities for pertechnetate. Adsorption kinetics of the pertechnetate ion on these materials is relatively fast and depends on pH. For some sorbents, a 99 %-adsorption within 1 min was found. One of the variables used to characterize of pertechnetate adsorption is distribution coefficient Kd. Maximum Kd of about 7 × 104 mL g−1 was measured for acidic pH (pH 2–3). In general, Kd was decreasing with increasing pH; however, the sample treated with zinc chloride sorbed TcO4 − very well even at pH 8 (Kd = 5 × 103 mL g−1).











Similar content being viewed by others
References
Perrier C, Segrè E (1937) Some chemical properties of element 43. J Chem Phys 5:712–716
Kónya J, Nagy NM (2012) Nuclear and radiochemistry. Elsevier, Amsterdam
Lieser KH (1993) Technetium in the nuclear fuel cycle, in medicine and in the environment. Radiochim Acta 63:5–8
Desmet G, Myttenaere C (1986) Technetium in the environment. Elsevier, Amsterdam
Schwochau K (2000) Technetium: chemistry and radiopharmaceutical applications. Wiley-VCH, Weinheim
Brookins DG (1988) Eh-pH diagrams for geochemistry. Springer, Berlin
Poineau F, Mausolf E, Jarvinen GD, Sattelberger AP, Czerwinski KR (2012) Technetium chemistry in the fuel cycle: combining basic and applied studies. Inorg Chem 51(15):8462–8467
El-Wear S, German KE, Peretrukhin VF (1992) Sorption of technetium on inorganic sorbents and natural minerals. J Radioanal Nucl Chem 157(1):3–14
Jedináková-Křížová V (1996) Radionuclides migration in the geosphere and their sorption on natural sorbents. J Radioanal Nucl Chem 208(2):559–575
Kohličková M, Jedináková-Křížová V, Horejš M (1999) Influence of technetium and rhenium speciation on their sorption on natural sorbents. Czech J Phys 49(1):695–700
Tkáč P, Kopunec R, Macášek F, Skrašková S (2000) Sorption of Tc(IV) and Tc(VII) on soils: influence of humic substances. J Radioanal Nucl Chem 246(3):527–531
Vinšová H, Konirová R, Koudelková M, Jedináková-Křížová V (2004) Sorption of technetium and rhenium on natural sorbents under aerobic conditions. J Radioanal Nucl Chem 261(2):407–413
Večerník P, Jedináková-Křížová V (2006) Diffusion of 99-technetium in compacted bentonite under aerobic and anaerobic conditions. Czech J Phys 56:D665–D672
Mattson JS, Mark HB Jr (1971) Activated carbon. Dekker, New York
Hayashi J, Kazehaya A, Muroyama K, Watkinson AP (2000) Preparation of activated carbon from lignin by chemical activation. Carbon 38:1873–1878
Marsh H (2001) Activated carbon compendium. Elsevier, Amsterdam
Hu Z, Srinivasan MP (2001) Mesoporous high-surface-area activated carbon. Microporous Mesoporous Mater 43:267–275
Bansal RCh, Goyal M (2005) Activated carbon adsorption. Taylor and Francis, London
Kang S, Jian-Chun J, Dan-dan C (2011) Preparation of activated carbon with highly developed mesoporous structure from Camellia oleifera shell through water vapor gasification and phosphoric acid modification. Biomass Bioenergy 35:3643–3647
Giraldo L, Moreno-Piraján CM (2012) Synthesis of activated carbon mesoporous from coffee waste and its application in adsorption zinc and mercury ions from aqueous solution. J Chem 9(2):938–948
Stoeckli F (1990) Microporous carbons and their characterization: the present state of art. Carbon 28:1–6
Rodriguez-Reinoso F, Linares-Solano A (1989) In: Thrower PA (ed) Chemistry and physics of carbon: a series of advances. Marcel Dekker, New York
Chmielewska E (2008) Development of new generation of environmental adsorbents based on natural nanomaterials. Chem Listy 102(2):124–130
McDougall GJ, Hancock RD, Nicol MJ, Wellington OL, Copperthwaite RG (1980) The mechanism of the adsorption of gold cyanide on activated carbon. J S Afr Inst Min Metall 80:344–356
Al-Bayoumy S, El-Kolaly M (1982) Some radiochemical studies on the adsorption behaviour of molybdenum-99 on silver-coated carbon granules and activated carbon. J Radioanal Nucl Chem 68(1):7–13
Morozova AA, Shashkova IL (1995) Sorption of cesium, strontium, and some toxic metal ions from aqueous media on chemically modified carbon sorbents. Pharm Chem J 29(8):553–556
Mandić M, Vuković Ž, Lazić S, Raičević S (1996) Sorption of hypoiodous acid on activated carbon. J Radioanal Nucl Chem 208(2):453–460
Wu J, Xie Z, Guo K, Claesson O (2001) Measurement and prediction of the adsorption of binary mixtures of organic vapors on activated carbon. Adsorpt Sci Technol 19:737–749
Wu J, Hammarström L-G, Claesson O, Fängmark IE (2003) Modeling the influence of physico-chemical properties of volatile organic compounds on activated carbon adsorption capacity. Carbon 41:1322–1325
Pawłowski L, Dudzinska MR, Pawłowski A (2003) In: Wolborska A, Pilecka-Bujnowicz K (eds) Environmental engineering studies. Kluwer Academic/Plenum Publishers, New York
Wu J (2004) Modeling adsorption of organic compounds on activated carbon A multivariate approach. Solfjädern Offset AB, Umeå
Samonin VV, Nikonova VYu, Podvyaznikov ML (2008) Sorption properties of fullerene-modified activated carbon with respect to metal ions. Prot Met 44(2):190–192
Tikhonova LP, Goba VE, Kovtun MF, Tarasenko YuA, Khavryuchenko VD, Lyubchik SB, Boiko AN (2008) Sorption of metal ions from multicomponent aqueous solutions by activated carbons produced from waste. Russ J Appl Chem 81(8):1348–1355
Buzaeva MV, Kalyukova EN, Klimov ES (2010) Sorption properties of AG-3 activated carbon in relation to oil products. Russ J Appl Chem 83(10):1883–1885
Sheveleva IV, Zheleznov VV, Bratskaya SYu, Avramenko VA, Kuryavyi VG (2011) Sorption of cesium radionuclides with composite carbon fibrous materials. Russ J Appl Chem 84(7):1152–1157
Bazan RE, Bastos-Neto M, Moeller A, Dreisbach F, Staudt R (2011) Adsorption equilibria of O2, Ar, Kr and Xe on activated carbon and zeolites: single component and mixture data. Adsorption 17:371–383
Sheng G, Li Y, Dong H, Shao D (2012) Environmental condition effects on radionuclide 64Cu(II) sequestration to a novel composite: polyaniline grafted multiwalled carbon nanotubes. J Radioanal Nucl Chem 293(3):797–806
Kaźmierczak J, Nowicki P, Pietrzak R (2012) Sorption properties of activated carbons obtained from corn cobs by chemical and physical activation. Adsorption 19:273–281
Fang Q, Chen B (2012) Adsorption of perchlorate onto raw and oxidized carbon nanotubes in aqueous solution. Carbon 50:2209–2219
Yoon I-H, Meng X, Wang Ch, Kim K-W, Bang S, Choe E, Lippincott L (2009) Perchlorate adsorption and desorption on activated carbon and anion exchange resin. J Hazard Mater 164:87–94
Weber W Jr (1974) Adsorption processes. Pure Appl Chem 37(3):375–392
Lin Ch-Ch (1979) Kinetics and mechanisms of adsorption of heavy metal ions on activated carbon, (Thesis). https://repositories.tdl.org/ttuir/bitstream/handle/2346/21688/31295001871556.pdf?sequence=1
Cheremisionoff NP, Ellerbusch F (1978) Carbon adsorption handbook. Ann Arbor Science Publishers, Michigan
Stoeckli F, Hugi-Cleary D (2001) On the mechanisms of phenol adsorption by carbons. Russ Chem Bul 50(11):2060–2063
Kawasaki M, Omori T, Hasegawa K (1993) Adsorption of pertechnetate on an anion exchange resin. Radiochim Acta 63:53–56
Wang Y, Gao H, Yeredla R, Xu H, Abrecht M (2007) Control of pertechnetate sorption on activated carbon by surface functional groups. J Coll Inter Sci 305:209–217
Lodewyckx P (2008) Adsorption on activated carbon: one underlying mechanism? NATO science for peace and security series C: environmental security. Springer, Netherlands
Viani B (1999) Assessing materials (“Geters”) to immobilize or retard the transport of technetium trough the engineered barrier system at the potential Yucca mountain nuclear waste repository. Lawrence Livermore National Laboratoy, Livermore
Poineau F, Hartmann T, Chinthaka Silva GW, Jarvinen G, Czerwinski K (2009) Preparation of technetium metal by thermal treatment under argon/H2O. J Radioanal Nucl Chem 279(1):16
Yamagishi I, Kubota M (1989) Separation of technetium with active carbon. Nucl Sci Technol 26:1038–1044
Gu B, Dowlen KE, Liang L, Clausen JL (1996) Efficient separation and recovery of technetium-99 from contaminated groundwater. Sep Technol 6:123–132
Holm E, Gäfvert T, Lindhal P, Roos P (2000) In situ sorption of technetium using activated carbon. Appl Radiat Isot 53:153–157
Ito K, Akiba K (1991) Adsorption of pertechnetate ion on active carbon from acids and their salt solutions. J Radioanal Nucl Chem 152:381–390
Ito K, Yachidate A (1992) Behavior of pertechnetate ion adsorption from aqueous solutions shown by activated carbons derived from different solutions. Carbon 30(5):767–771
Harkins WD, Jura EJ (1944) The decrease of free surface energy as a basis for the development of equations for adsorption isotherms; and the existence of two condensed phases in films on solids. J Chem Phys 12:112–113
Barrett EPT, Joyner LG, Halenda PP (1951) The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J Am Chem Soc 73:373–380
ImageJ Application (2004) National Institutes of Health, Maryland. http://rsb.info.nih.gov/ij/plugins/
Venhryn BYA, Grygorchak II, Kulyk YUO, Mudry SI, Shvets RYA (2008) Porous structure of carbon-based materials studied by means of X-ray small angle scattering method. Opt Appl 38(1):119–124
Sing KSW, Everett DH, Haul RAW, Moscou L, Pierotti RA, Roquérol J, Siemieniewska T (1985) Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendation 1984, IUPAC, Physical Chemistry Division). Pure Appl Chem 57(4):603–619
Hudec P (2012) Texture of solids—determination of surface properties of adsorbents and catalysts by physical adsorption of nitrogen (in Slovak). STU publisher, Bratislava
Groen JC, Peffer LAA, Pérez-Ramírez J (2003) Pore size determination in modified micro- and mesoporous materials Pitfalls and limitations in gas adsorption data analysis. Microporous Mesoporous Mater 60(1):1–17
Matisová E, Hrouzková S, Novák I, Berek D, Kozánková J (1999) Novel porous carbons and their utlization in trace analysis. Chem Papers 53(1):40–48
Acknowledgments
This contribution/publication is the result of the project implementation: CE for development and application of advanced diagnostic methods in processing of metallic and non-metallic materials, ITMS: 26220120048, supported by the Research and Development Operational, Program funded by the ERDF.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rajec, P., Galamboš, M., Daňo, M. et al. Preparation and characterization of adsorbent based on carbon for pertechnetate adsorption. J Radioanal Nucl Chem 303, 277–286 (2015). https://doi.org/10.1007/s10967-014-3303-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-014-3303-y