Abstract
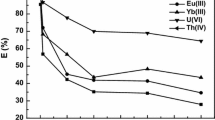
In this study, extraction and separation of thorium(IV) and a few representative rare earths in HNO3 media was evaluated using trioctylmethylammonium nitrate ([A336][NO3]) ionic liquid. An unexpected novel extraction mechanism was identified based on the studies in the slope analysis and ESI–MS spectrum. The trimer of [A336][NO3] was confirmed to dominate the extraction reaction by ESI–MS spectrum before and after the extraction. The extraction efficiency of La(III), Eu(III) and Lu(III) by [A336][NO3] are much lower than Th(IV) in acid medium, which means that it is possible to separate thorium from rare earths or the rare earth ores by using [A336][NO3].










Similar content being viewed by others
References
Nasab ME (2014) Solvent extraction separation of uranium(VI) and thorium(IV) with neutral organophosphorus and amine ligands. Fuel 116:595–600
Rao A, Tomar BS (2016) Extraction of thorium employing N,N-dialkyl amide into room temperature ionic liquid followed by supercritical carbon dioxide stripping. Sep Purif Technol 161:159–164
Ashley SF, Parks GT, Nuttall WJ, Boxall C, Grimes RW (2012) Thorium fuel has risks. Nature 492:31–33
Li Z, Zhao H, He SH, Chen M, Li QA (2016) Systematic research on solvent extraction process for extracting 233U from irradiated thorium. Hydrometallurgy 166:160–166
Nasab ME, Sam A, Milani SA (2011) Determination of optimum process conditions for the separation of thorium and rare earth elements by solvent extraction. Hydrometallurgy 106:141–147
Kuang S, Zhang Z, Li Y, Wu G, Wei H, Liao W (2017) Selective extraction and separation of Ce(IV) from thorium and trivalent rare earths in sulfate medium by an α-aminophosphonate extractant. Hydrometallurgy 167:107–114
Yan Z-Y, Huang Q-G, Wang L, Zhang F (2019) Synthesis of tailored bis-succinamides and comparison of their extractability for U(VI), Th(IV) and Eu(III). Sep Purif Technol 213:322–327
Raju CSK, Subramanian MS (2007) Sequential separation of lanthanides, thorium and uranium using novel solid phase extraction method from high acidic nuclear wastes. J Hazard Mater 145:315–322
McCann K, Mincher BJ, Schmitt NC, Braley JC (2017) Hexavalent actinide extraction using N,N-dialkyl amides. Ind Eng Chem Res 56:6515–6519
Ren P, Wang C, Tao W, Yang X, Yang S, Yuan L, Chai Z, Shi W (2020) Selective separation and coordination of europium(III) and americium(III) by bisdiglycolamide ligands: solvent extraction, spectroscopy, and DFT calculations. Inorg Chem 59:14218–14228
Ibrahim SM, Zhang Y, Xue Y, Yang S, Ma F, Tian G (2020) Extraction of lanthanides(III) along with thorium(IV) from chloride solutions by N,N-di(2-Ethylhexyl)-diglycolamic acid. Solvent Extr Ion Exc 38:417–429
Rout A, Venkatesan KA, Srinivasan TG, Rao PRV (2012) Ionic liquid extractants in molecular diluents: extraction behavior of europium(III) in quarternary ammonium-based ionic liquids. Sep Purif Technol 95:26–31
Zuo Y, Chen J, Li DQ (2008) Reversed micellar solubilization extraction and separation of thorium(IV) from rare earth(III) by primary amine N1923 in ionic liquid. Sep Purif Technol 63:684–690
Gupta B, Malik P, Deep A (2002) Extraction of uranium, thorium and lanthanides using Cyanex-923: their separations and recovery from monazite. J Radioanal Nucl Chem 251:451–456
Nanda D, Oak MS, Maiti B, Chauhan HP, Dutta PK (2002) Selective and uphill transport of uranyl ion in the presence of some base metals and thorium across bulk liquid membrane by di (2-ethylhexyl) phosphoric acid. Sep Sci Technol 37:3357–3367
Niu Y-N, Ren P, Zhang F, Yan Z-Y (2018) Solvent extraction of Eu3+ with 4-oxaheptanediamide into ionic liquid system. Sep Sci Technol 53:2750–2755
Errico M, Eduardo SR, Juan JQR, Rong BG, Juan GSH (2017) Multi objective optimal acetone-butanol-ethanol (ABE) separation systems using liquid–liquid extraction assisted divided wall columns. Ind Eng Chem Res 56:11575–11583
Yan Z-Y, Ren P, Huang Q-G, He M-J, Li Y, Wu Z-M, Wu W-S (2016) Solvent extraction of uranyl ion with 4-oxaheptanediamide into ionic liquid system from HNO3 solution. J Radioanal Nucl Chem 310:703–709
Dai S, Luo J, Li J, Zhu X, Cao Y, Komarneni S (2017) Liquid-liquid micro-extraction of Cu2+ from water using a new circle micro-channel device. Ind Eng Chem Res 56:12717–12725
Shkrob IA, Marin TW, Jensen MP (2014) Ionic liquid based separations of trivalent lanthanide and actinide. Ind Eng Chem Res 53:3641–3653
Depuydt D, Dehaen W, Binnemans K (2015) Solvent extraction of scandium(III) by an aqueous biphasic system with a nonfluorinated functionalized ionic liquid. Ind Eng Chem Res 54:8988–8996
Sun X, Luo H, Dai S (2011) Ionic liquids-based extraction: a promising strategy for the advanced nuclear fuel cycle. Chem Rev 112:2100–2128
Zhu M, Zhao J, Li Y, Mehio N, Qi Y, Liu H, Dai S (2015) An ionic liquid-based synergistic extraction strategy for rare earths. Green Chem 17:2981–2993
Leyma R, Platzer S, Jirsa F, Kandioller W, Krachler R, Keppler BK (2016) Novel thiosalicylate-based ionic liquids for heavy metal extractions. J Hazard Mater 314:164–171
Hoogerstraete TV, Binnemans K (2014) Highly efficient separation of rare earths from nickel and cobalt by solvent extraction with the ionic liquid trihexyl(tetradecyl)phosphonium nitrate: process relevant to the recycling of rare earths from permanent magnets and nickel metal hydride batteries. Green Chem 16:1594–1606
Hoogerstraete TV, Wellens S, Verachtert K, Binnemans K (2013) Removal of transition metals from rare earths by solvent extraction with an undiluted phosphonium ionic liquid: separations relevant to rare-earth magnet recycling. Green Chem 15:919–927
De los Ríos AP, Hernández-Fernández FJ, Alguacil FJ, Lozano LJ, Ginestá A, García-Díaz I, Sánchez-Segado S, López FA, Godínez C (2012) On the use of imidazolium and ammonium-based ionic liquids as green solvents for the selective recovery of Zn(II), Cd(II), Cu(II) and Fe(III) from hydrochloride aqueous solutions. Sep Purif Technol 97:150–157
Sun T, Xu C, Fu J, Chen Q, Chen J, Shen X (2017) Extraction of U(VI) by the ionic liquid hexyltributylphosphonium bis (trifluoromethylsulfonyl)imides: an experimental and theoretical study. Sep Purif Technol 188:386–393
Acknowledgements
The authors thank the National Natural Science Foundation of China (Grant No. 21471072) for the financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fu, X., Zhang, F., Wu, Q. et al. The separation of thorium and rare earth elements using [A336][NO3]: insight into a new extraction mechanism. J Radioanal Nucl Chem 327, 1251–1258 (2021). https://doi.org/10.1007/s10967-020-07590-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-020-07590-y