Abstract
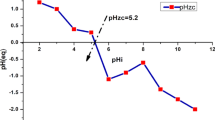
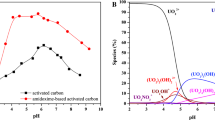
Phosphorylated activated carbon was prepared from silk gourd loofah rattan by phosphoric acid modification. The effect of pH value, original content of uranyl ions, time and temperature on the adsorption of uranium by phosphorylated activated carbon were studied by static experiments. In this paper, the adsorption process was analyzed by thermodynamics, kinetics and isotherm model. The adsorbent was characterized using infrared spectroscopy and scanning electron microscopy. The results showed that the pH of the solution can significantly affect the adsorption performance and the pH value of the best adsorption was 5.0. The adsorption equilibrium time was about 240 min and when the adsorption quantity was 197 mg/g, it reaches the summit, and the pseudo-second-order equation and Langmuir equation were more suitable to describe the adsorption process.












Similar content being viewed by others
References
Neusatz Guilhen S, Rovani S, Pitol Filho L, Alves Fungaro D (2019) Kinetic study of uranium removal from aqueous solutions by macaúba biochar. Chem Eng Commun 206(11):1354–1366
Corner A, Venables D, Spence A, Poortinga W, Demski C, Pidgeon N (2011) Nuclear power, climate change and energy security: exploring British public attitudes. Energy Policy 39(9):4823–4833
Yu SQ, Wang XX, Ning SY, Chen ZS, Wang XK (2019) Highly efficient carbonaceous nanofiber/layered double hydroxide nanocomposites for removal of U(VI) from aqueous solutions. Radiochim Acta 107(4):299–309
Zhong CN, Su SZ, Xu L, Liu Q, Zhang HS, Yang PP, Zhang ML, Bai XF, Wang J (2019) Preparation of NiAl-LDH/polypyrrole composites for uranium(VI) extraction from simulated seawater. Colloid Surf A 562:329–335
Li P, Chen P, Liu ZP, Nie SY, Wang XG, Wang GH, Zhang WM, Chen H, Wang LZ (2020) Highly efficient elimination of uranium from wastewater with facilely synthesized Mg–Fe layered double hydroxides: optimum preparation conditions and adsorption kinetics. Ann Nucl Energy. https://doi.org/10.1016/j.anucene.2019.107140
Bajwa B, Kumar S, Singh S, Sahoo S, Tripathi R (2017) Uranium and other heavy toxic elements distribution in the drinking water samples of SW-Punjab, India. J Radiat Res Appl Sci 10(1):13–19
Atia BM, Gado MA, Abd El-Magied MO, Elshehy EA (2020) Highly efficient extraction of uranyl ions from aqueous solutions using multi-chelators functionalized graphene oxide. Sep Sci Techol 15(55):2746–2757
Chen T, Zhang J, Ge H, Li M, Li Y, Duan T, He R, Zhu W (2020) Efficient extraction of uranium in organics-containing wastewater over g-C3N4/GO hybrid nanosheets with type-II band structure. J Hazard Mater 384:121383
Zhu JH, Zhang HS, Chen RR, Liu Q, Liu JY, Yu J, Li RM, Zhang ML, Wang J (2019) An anti-algae adsorbent for uranium extraction: l-arginine functionalized graphene hydrogel loaded with Ag nanoparticles. Colloid Surf A 543:192–200
Zhao K, Chen C, Cheng T, Shang JY (2019) Graphene oxide-facilitated uranium transport and release in saturated medium: effect of ionic strength and medium structure. Environ Pollut 247:668–677
Chen MM, Li Z, Geng YY, Zhao HG, He SH, Li QN, Zhang L (2018) Adsorption behavior of thorium on N,N,N′,N′-tetraoctyldiglycolamide (TODGA) impregnated graphene aerogel. Talanta 181:311–317
Wang X, Liu Q, Liu JY, Chen RR, Zhang HS, Li RM, Li ZS, Wang J (2017) 3D self-assembly polyethyleneimine modified graphene oxide hydrogel for the extraction of uranium from aqueous solution. Appl Surf Sci 426:1063–1074
Liu X, Sun J, Xu XT, Alsaedi A, Hayat T, Li JX (2019) Adsorption and desorption of U(VI) on different-size graphene oxide. Chem Eng J 360:941–950
Yu KF, Shao PF, Meng PW, Chen T, Lei J, Yu XF, He R, Yang F, Zhu WK, Duan T (2020) Superhydrophilic and highly elastic monolithic sponge for efficient solar-driven radioactive wastewater treatment under one sun. J Hazard Mater 392:122350
Li RW, Ibeanusi V, Hoyle-Gardner J, Crandall C, Jagoe C, Seaman J, Anandhi A, Chen G (2019) Bacterial-facilitated uranium transport in the presence of phytate at Savannah River Site. Chemosphere 223:351–357
Manoj S, Thirumurugan M, Elango L (2020) Determination of distribution coefficient of uranium from physical and chemical properties of soil. Chemosphere 244:263–271
Yang DS, Xu BC, Burnett W, Yu ZG, Jiang XY, Zhang XJ, Zhao SB, Xia D (2019) Radium isotopes-suspended sediment relationships in a muddy river. Chemosphere 214:250–258
Sun Y, Kang Y, Zhong W, Liu Y, Dai Y et al (2020) A simple phosphorylation modification of hydrothermally cross-linked chitosan for selective and efficient removal of U(VI). J Soild State Chem 292:121731
Sha TZ, Liu JJ, Sun MM, Li L, Bai J, Hu ZQ, Zhou M (2019) Green and low-cost synthesis of nitrogen-doped graphene-like mesoporous nanosheets from the biomass waste of okara for the amperometric detection of vitamin C in real samples. Talanta 200:300–306
Liao Y, Wang M, Chen DJ (2019) Electrosorption of uranium(VI) by highly porous phosphate-functionalized graphene hydrogel. Appl Surf Sci 484:83–96
Liu W, Wang YL, Song LP, Silver MA, Xie J, Zhang LM, Chen LH, Juan DW, Chai ZF, Wang SA (2019) Efficient and selective sensing of Cu2+ and UO22+ by a europium metal-organic framework. Talanta 196:515–522
Yang S, Zhang XW, Wu XY, Li M, Zhang LJ, Peng Y, Huang QW, Tan WF (2019) Understanding the solid phase chemical fractionation of uranium in soil profile near a hydrometallurgical factory. Chemosphere 236:323–336
Yin ML, Tsang DCW, Sun J, Wang J, Shang JY, Fang F, Wu Y, Liu J, Song G, Xiao TF, Chen DY (2020) Critical insight and indication on particle size effects towards uranium release from uranium mill tailings: Geochemical and mineralogical aspects. Chemosphere 250:287–298
Kirby ME, Watson JS, Najorka J, Kenney JPL, Krevor S, Weiss DJ (2020) Experimental study of pH effect on uranium(U-VI) particle formation and transport through quartz sand in alkaline 0.1 M sodium chloride solutions. Colloid Surface A 592:235–241
Sassolas-Serrayet T, Cattin R, Ferry MA, Godard V, Simoes M (2019) Estimating the disequilibrium in denudation rates due to divide migration at the scale of river basins. Earth Surf Dyn 7(4):1041–1057
Chen C, Zhao K, Shang JY, Liu CX, Wang J, Yan ZF, Liu KS, Wu WL (2018) Uranium(VI) transport in saturated heterogeneous media: Influence of kaolinite and humic acid. Environ Pollut 240:219–226
Liatsou I, Michail G, Demetriou M, Pashalidis I (2017) Uranium binding by biochar fibres derived from Luffa cylindrica after controlled surface oxidation. J Radioanal Nucl Chem 311(1):871–875
Yousef LA, Morsy AMA, Hagag MS (2020) Uranium ions adsorption from acid leach liquor using acid cured phosphate rock: kinetic, equilibrium, and thermodynamic studies. Sep Sci Technol 55(4):648–657
Wang M, Cheng W, Wan T, Hu BW, Zhu YL, Song XF, Sun YB (2019) Mechanistic investigation of U(VI) sequestration by zero-valent iron/activated carbon composites. Chem Eng J 362:99–106
Hu R, Xiao J, Wang TH, Chen GC, Chen L, Tian XY (2020) Engineering of phosphate-functionalized biochars with highly developed surface area and porosity for efficient and selective extraction of uranium. Chem Eng J 379:122388
Zhang ZB, Dong ZM, Wang XX, Ying D, Niu FL, Cao XH, Wang YQ, Hua R, Liu YH, Wang XK (2018) Ordered mesoporous polymer-carbon composites containing amidoxime groups for uranium removal from aqueous solutions. Chem Eng J 341:208–217
Ye TZ, Liu ZR, Cai ZW (2020) Adsorption of uranium(VI) from aqueous solution by novel dibutyl imide chelating resin. J Radioanal Nucl Chem 323(1):223–232
Yang S, Xu MY, Yin J, Zhao TX, Li CQ, Hua DB (2020) Thermal-responsive Ion-imprinted magnetic microspheres for selective separation and controllable release of uranium from highly saline radioactive effluents. Sep Purif Technol 246:8
Singhal P, Vats BG, Yadav A, Pulhani V (2020) Efficient extraction of uranium from environmental samples using phosphoramide functionalized magnetic nanoparticles: understanding adsorption and binding mechanisms. J Hazard Mater 384:121353
Basu H, Pimple MV, Saha S, Patel A, Dansena C, Singhal RK (2020) TiO2 microsphere impregnated alginate: a novel hybrid sorbent for uranium removal from aquatic bodies. New J Chem 44(10):3950–3960
Acknowledgements
This work was supported by the National Natural Science Fund Program (11875105, 21866006); the Main Academic and Technology Leader Funding Program of Jiangxi Province(20172BCB22020); the Science and Technology Pillar Program of Jiangxi Province(20192BBG70062).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, Y., Ye, T., Wang, Y. et al. Adsorption of uranium(VI) from aqueous solution by phosphorylated luffa rattan activated carbon. J Radioanal Nucl Chem 327, 1267–1275 (2021). https://doi.org/10.1007/s10967-020-07592-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-020-07592-w