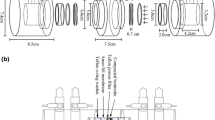
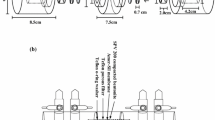
Abstract
Diffusion is the dominant process involved in the transport of 129I through geological media; therefore, the evaluation of the experimental results on the diffusion of I− through compacted bentonite was performed by three diffusion models, CC-CC, CC-VC, and VC-VC. Multiple experiments were cross-checked to verify internal consistency and thereby obtain reliable diffusion parameters. The results showed that I− diffusion coefficients ranged from 1.15 × 10–12 to 4.67 × 10–12 m2/s, which were consistent with those of the literature. These techniques could potentially be performed to measure the diffusion coefficients of non-sorbing or weakly sorbing radionuclides onto compacted bentonite in radioactive waste repositories.





Similar content being viewed by others
Abbreviations
- αc :
-
Capacity factor of porous medium, dimensionless
- ρb :
-
Bulk dry density of the porous medium, gcm−3
- θtot :
-
Total porosity of the porous medium; dimensionless
- Kd :
-
Distribution coefficient, cm3 g−1
- A:
-
Cross sectional area of specimen normal to the direction of the diffusional flux, cm2
- L:
-
Thickness (or length) of the specimen in the direction of diffusion, cm
- C:
-
Concentration of the diffusing substance in pore water, ppm
- CA :
-
Concentration of the diffusing substance in the inlet reservoir, ppm
- CB :
-
Concentration of the diffusing substance in the outlet reservoir, ppm
- Qt :
-
Total accumulated amount of diffusing substance, mol
- Da :
-
Apparent diffusion coefficient, m2 s-1
- De :
-
Effective diffusion coefficient, m2 s-1
- VA :
-
Volume of inlet reservoir, mL
- VB :
-
Volume of outlet reservoir, mL
- V1 :
-
Volume of specimen, mL
- S:
-
Fitted slope
- t:
-
Time, s
- tx :
-
Time-lag, s
References
Kuo CL, Tsai TL, Chiang A, Chang-Liao KS, Chao JH (2013) Determination of 129I in cement-solidified radwastes using neutron activation. J Radioanal Nucl Chem 298:465–473. https://doi.org/10.1007/s10967-013-2486-y.pdf
Palágyi Š, Štamberg K (2014) Transport parameters of I-and IO3- determined in crushed granitic rock columns and groundwater system under dynamic flow conditions. J Radioanal Nucl Chem 302:647–653. https://doi.org/10.1007/s10967-014-3267-y
Santschi PH, Xu C, Zhang S, Schwehr KA, Grandbois R, Kaplan DI, Yeager CM (2017) Iodine and plutonium association with natural organic matter: A review of recent advances. Appl Geochem 85:121–127. https://www.sciencedirect.com/science/article/pii/S0883292716303766
Zhang S, Du J, Xu C, Schwehr KA, Ho YF, Li HP, Roberts KA, Kaplan DI, Brinkmeyer R, Yeager CM, Chang HS, Santschi PH (2011) Concentration dependent mobility, retardation and speciation of iodine in surface sediment from the Savannah River Site. Environ Sci Technol 45:5543–5549. https://doi.org/10.1021/es1040442
Kaplan DI, Serne RJ, Parker KE, Kutnyakov IV (2000) Iodide sorption to subsurface sediments and illite minerals. Environ Sci Technol 34:399–405. https://doi.org/10.1021/es990220g
Aimoz L, Curti E, Mäder U (2011) Iodide interaction with natural pyrite. J Radioanal Nucl Chem 288:517–524. https://doi.org/10.1007/s10967-010-0959-9
Lee JO, Lee KJ (1997) Sorption and diffusion of 1–125 and Sr-90 in a mixture of Bentonite and crushed granite backfill of a radioactive waste repository. Radiochim Acta 76:143–151. https://doi.org/10.1524/ract.1997.76.3.143
Eriksen TE, Jansson M, Molera M (1999) Sorption effects on cation diffusion in compacted bentonite. Eng Geol 54(1–2):231–236. https://doi.org/10.1016/S0013-7952(99)00078-2
Tsai SC, Ouyang S, Hsu CN (2001) Sorption and diffusion behavior of Cs and Sr on Jih-Hsing bentonite. Appl Radiat Isot 54(2):209–215. https://doi.org/10.1016/s0969-8043(00)00292-x
Szántó Z, Svingor É, Molnár M, Palcsu L, Futó I, Szűcs Z (2002) Diffusion of H-3, Tc-99, I-125, Cl-36 and Sr-85 in granite, concrete and bentonite. J Radioanal Nucl Chem 252:133–138. https://doi.org/10.1023/A:1015256308843
Van Loon LR, Glaus MA, Müller W (2007) Anion exclusion effects in compacted bentonites: towards a better understanding of anion diffusion. Appl Geochem 22(11):2536–2552. https://doi.org/10.1016/j.apgeochem.2007.07.008
García-Gutiérrez M, Cormenzana JL, Missana T, Mingarro M (2004) Diffusion coefficients and accessible porosity for HTO and 36Cl in compacted FEBEX bentonite. Appl Clay Sci 26:65–73. https://doi.org/10.1016/j.clay.2003.09.012
Tsai TL, Tsai SC, Shih YH, Chen LC, Lee CP, Su TY (2017) Diffusion characteristics of HTO and 99TcO4− in compacted Gaomiaozi (GMZ) bentonite. Nucl Sci Tech 28:67. https://doi.org/10.1007/s41365-017-0221-z
Shih YH, Lee IH, Ni CF, Tsai TL, Chen LC, Lee CP, Tsai SC, Su TY (2018) Experimental and numerical investigations of 99TcO4− diffusion in compacted SPV 200 bentonite. J Radioanal Nucl Chem 316:1081–1089. https://doi.org/10.1007/s10967-018-5800-x
Bourg IC, Sposito G, Bourg ACM (2006) Tracer diffusion in compacted, water saturated bentonites. Clays Clay Miner 54(3):363–374. https://doi.org/10.1346/CCMN.2006.0540307
Wang X, Tao Z (2004) Diffusion of 99TcO4- in compacted bentonite: Effect of pH, concentration, density and contact time. J Radioanal Nucl Chem 260(2):305–309. https://doi.org/10.1023/B:JRNC.0000027101.01834.1b.pdf
Tian W, Li C, Liu X, Wang L, Zheng Z, Wang X, Liu C (2013) The effect of ionic strength on the diffusion of 125I in Gaomiaozi bentonite. J Radioanal Nucl Chem 295:1423–1430. https://doi.org/10.1007/s10967-012-2284-y
Ishidera T, Miyamoto S, Sato H (2008) Effect of Sodium Nitrate on the Diffusion of Cl- and I- in Compacted Bentonite. J Nucl Sci Technol 45(7):610–616. https://doi.org/10.1080/18811248.2008.9711459
Shackelford CD (1991) Laboratory diffusion testing for waste disposal - A review. J Contam Hydrol 7(3):177–217. https://doi.org/10.1016/0169-7722(91)90028-Y
Lever DA (1986) Some notes on experiments measuring diffusion of sorbed nuclides through Porous Media. AERE-R-12321. United Kingdom Atomic Energy Authority, Publications Office, Harwell Laboratory, Oxfordshire OX11 0RA, England.
García-Gutiérrez M, Cormenzana JL, Missana T, Mingarro M, Molinero J (2006) Overview of laboratory methods employed for obtaining diffusion coefficients in FEBEX compacted bentonite. J Iber Geol 32(1):37–53. https://www.ucm.es/info/estratig/journal.htm
Aldaba D, Rigol A, Vidal M (2010) Diffusion experiments for estimating radiocesium and radiostrontium sorption in unsaturated soils from Spain: Comparison with batch sorption data. J Hazard Mater 181(1–3):1072–1079. https://doi.org/10.1016/j.jhazmat.2010.05.124
Buil B, Gomez P, Pena J, Garralon A, Turrero MJ, Escribano A, Sanchez L, Duran JM (2010) Modelling of bentonite–granite solutes transfer from an in situ full-scale experiment to simulate a deep geological repository (Grimsel Test Site, Switzerland). Appl Geochem 25(12):1797–1804. https://doi.org/10.1016/j.apgeochem.2010.09.003
Savoye S, Page J, Puente C, Imbert C, Coelho D (2010) New experimental approach for studying diffusion through an intact and unsaturated medium: A case study with Callovo-Oxfordian Argillite. Environ Sci Technol 44:3698–3704. https://doi.org/10.1021/es903738t
Carslaw HS, Jaeger JC (1959) Conduction of heat in solids, 2nd edn. Oxford University, New York
Crank J (1975) The mathematics of diffusion, 2nd edn. Clarendon Press, Oxford
Zhang M, Takeda M, Nakajima H (2006) Strategies for solving potential problems associated with laboratory diffusion and batch experiments-part 1. An overview of conventional test methods, in: WM'06 Conference, Tucson, AZ, https://www.osti.gov/biblio/21208622
Moridis GJ (1999) Semianalytical solutions for parameter estimation in diffusion cell experiments. Water Resour Res 35(6):1729–1740. https://doi.org/10.1029/1999WR900084
Bharat TV, Suvapullaiah PV, Allam MM (2009) Swarm intelligence-based solver for parameter estimation of laboratory through-diffusion transport of contaminants. Comput Geotech 36:984–992. https://doi.org/10.1016/j.compgeo.2009.03.006
Kong J, Lee CP, Sun YH, Hua R, Liu WG, Wang ZF, Li Y, Wang YD (2021) Anion exclusion and sorption effect for compacted bentonite: the dependency of diffusion coefficients and capacity of HTO and Se (IV). J Radioanal Nucl Chem 328:717–725. https://doi.org/10.1007/s10967-021-07688-x
Cheung SCH (1990) A new interpretation of measured ionic diffusion coefficient in compacted bentonite-based materials. Eng Geol 28(3–4):369–378. https://doi.org/10.1016/0013-7952(90)90021-R
Takeda M, Nakajima H, Zhang M, Hiratsuk T (2008) Laboratory longitudinal diffusion tests: 1. Dimensionless formulations and validity of simplified solutions. J Contam Hydrol 97:117–134. https://doi.org/10.1016/j.jconhyd.2008.01.004
Takeda M, Zhang M, Nakajima H (2006) Strategies for solving potential problems associated with laboratory diffusion and batch experiments- part 2. Future improvements, in: WM'06 Conference, Tucson, AZ, https://www.researchgate.net/publication/255209460
Zhang M, Takeda M, Nakajima H (2006) Determining the transport properties of rock specimens using an improved laboratory through-diffusion technique. MRS Online Proceedings of the 29th International Symposium on the Scientific Basis for Nuclear Waste Management. https://doi.org/10.1557/PROC-932-113.1
Chen CL, Wang TH, Lee CH, Teng SP (2012) The development of a through-diffusion model with a parent-daughter decay chain. J Contam Hydrol 138–139:1–14. https://doi.org/10.1016/j.jconhyd.2012.06.002
Hölttä P, Siitari-Kauppi M, Hakanen M, Tukiainen V (2001) Attempt to model laboratory- scale diffusion and retardation data. J Contam Hydrol 47(2–4):139–148. https://doi.org/10.1016/s0169-7722(00)00144-3
Chen CL, Wang TH, Lee CH, Teng SP (2014) Intercomparison of diffusion coefficient derived from the through diffusion experiment using different numerical methods. J Radioanal Nucl Chem 300: 393- 407. https://link.springer.com/article/https://doi.org/10.1007/s10967-014-2974-8
Li MH, Wang TH, Teng SP (2009) Experimental and numerical investigations of effect of column length on retardation factor determination: A case study of cesium transport in crushed granite. J Hazard Mater 162:530–535. https://doi.org/10.1016/j.jhazmat.2008.05.076
Van Loon LR, Soler JM, Bradbury MH (2003) Diffusion of HTO, 36Cl- and 125I- in Opalinus Clay samples from Mont Terri: effect of confining pressure. J Contam Hydrol 61(1–4):73–83. https://doi.org/10.1016/S0169-7722(02)00114-6
Lee JO, Cho WJ, Hahn PS, Park HH (1994) Diffusion characteristics of iodide in a domestic bentonite of Korea. J the Korean Nucl Soc 26(2):285–293. https://www.koreascience.or.kr/article/JAKO199411921629564.pdf
Shackelford CD, Moore SM (2013) Fickian diffusion of radionuclides for engineered containment barriers: Diffusion coefficients, porosities, and complicating issues. Eng Geol 152(1):133–147. https://doi.org/10.1016/j.enggeo.2012.10.014
Wu T, Wang Z, Wang H, Zhang Z, Van Loon LR (2017) Salt effects on Re (VII) and Se (IV) diffusion in bentonite. Appl Clay Sci 141:104–110. https://doi.org/10.1016/j.clay.2017.02.021
Appelo CAJ, Wersin P (2007) Multicomponent diffusion modeling in clay systems with application to diffusion of tritium, iodide, and sodium in Opalinus Clay. Environ Sci Technol 41(14):5002–5007. https://doi.org/10.1021/es0629256
Kozaki T, Inada K, Sato S, Ohashi H (2001) Diffusion mechanism of chloride ions in sodium montmorillonite. J Contam Hydrol 47(2–4):159–170. https://doi.org/10.1016/S0169-7722(00)00146-7
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tsai, TL., Tsai, SC., Chang, DM. et al. Intercomparison of determining diffusion coefficients of I− in compacted bentonite using various mathematical models of through-diffusion experiments in the laboratory. J Radioanal Nucl Chem 330, 1317–1327 (2021). https://doi.org/10.1007/s10967-021-08041-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-021-08041-y