Abstract
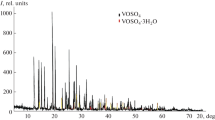
The kinetics of dehydration and decomposition of VOSO4·2H2O, VOSO4 and VOSeO3·H2O was studied under non-isothermal heating on a derivatograph. The stages and products of the thermal decomposition were determined. It was proved that VOSO4·2H2O decomposes to V2O5 while VOSeO3·H2O − to V2O4. A number of kinetic models and calculation procedures were used to determine the values of the kinetic parameters characterizing the process. The parameters calculated were compared and analyzed. IR-spectra of the initial substances and the solid residue after decomposition are presented.
Similar content being viewed by others
References
V. N. Muzgin, L. B. Hamzina, V. L. Zolotavin and I. Ya. Bezrukov, Analiticheskaya khimiya vanadiya, Nauka, Moscow, 1981.
R. Ripan and I. Chetyanu, Neorganicheskaya Khimiya, vol. 2, Mir, Moscow, 1972.
G. Meunier, M. Bertaund and J. Galy, Acta Crystallogr. B30 (1974) 2834.
J. C. J. Bart and G. Petrini, Z. Anorg. Allg. Chem., 422 (1976) 179.
J. C. Trombe, A. Gleizes and J. Galy, C. R. Acad. Sci. Ser. 2, 297 (1983) 667.
J. C. Trombe, A. Gleizes, J. Galy and J. P. Renard, Y. Journaux, New J. Chem., 11 (1987) 331.
G. Huan, J. W. Johnson, A. J. Jacobson, D. P. Goshorn and J. S. Merola, Chem. Mater., 3 (1991) 539.
W. T. A. Harrison, J. T. Vaughey, A. J. Jacobson, D. P. Goshorn and J. W. Johnson, J. Solid State Chem., 116 (1995) 77.
K. S. Lee, Y. U. Kown, Y. U. Kwon, H. Namgung and S. H. Kim, Inorg. Chem., 34 (1995) 4178.
W. T. A. Harrison, L. L. Dussac and A. J. Jacobson, Acta Crystallogr., Section C, 51 (1995) 24.
Y. H. Kim, Y. U. Kwon and K. S. Lee, Bull. Korean Chem. Soc., 17 (1996) 1123.
K. S. Lee and Y. U. Kwon, J. Korean Chem. Soc., 40 (1996) 379.
Y. U. Kwon, K. S. Lee and Y. H. Kim, Inorg. Chem., 35 (1996) 1161.
Y. H. Kim, K. S. Lee and Y. U. Kwon, Inorg. Chem., 35 (1996) 7394.
P. S. Halasyamani and D. O’Hare, Inorg. Chem., 36 (1997) 6409.
V. P. Verma, Thermochim. Acta, 327 (1999) 63 and references therein.
W. T. A. Harrison, Acta Cryst., Section C, 56 (2000) 422.
Y. T. Kim, Y. H. Kim, K. Park and Y. U. Kwon, J. Solid State Chem., 161 (2001) 23.
J. Šestak and G. Berggren, Thermochim. Acta, 3 (1971) 1.
C. L. Albano, R. Sciamanna, T. Aquino and J. Martinez, European Congress on Computational Methods in Applied Sciences and Enginering, (ECCOMAS 2000, Barcelona, 11–14 September 2000).
E. Tomaszewicz and M. Kotfica, J. Therm. Anal. Cal., 77 (2004) 25.
L. T. Vlaev, V. G. Georgieva and G. G. Gospodinov, J. Therm. Anal. Cal., 79 (2005) 163.
T. Wanjun, L. Yuwen, Y. Xil, W. Zhiyong and W. Cunxin, J. Therm. Anal. Cal., 81 (2005) 347
W. Coats and J. P. Redfern, Nature (London) 201, (1964) 68.
W. Tang, Y. Liu, H. Zang and C. Wang, Thermochim. Acta, 408 (2003) 39.
T. Wanjun, L. Yuwen, Z. Hen, W. Zhiyong and W. Cunxin, J. Therm. Anal. Cal., 74 (2003) 309.
L. T. Vlaev, I. G. Markovska and L. A. Lyubchev, Thermochim. Acta, 406 (2003) 1.
V. P. Verma and A. Khushu, J. Thermal Anal., 35 (1989) 1157.
L. T. Vlaev, S. D. Genieva and V. G. Georgieva, J. Therm. Anal. Cal., 86 (2006) 449.
L. T. Vlaev, S. D. Genieva and G. G. Gospodinov, J. Therm. Anal. Cal., 81 (2005) 469.
K. I. Petrov, I. V. Tananaev, A. N. Volodina and N. K. Bol’shakova, Zh. Neorg. Khim., 22 (1977) 1453.
R. Ya. Melnikovskii, V. N. Makatun and V. V. Pechkovskii, Zh. Neorg. Khim., 19 (1974) 1864. (in Russian).
R. C. Cody, Levitt, R. S. Viswanath and P. J. Miller, J. Solid State Chem., 26 (1978) 281.
M. Ebert, Z. Mička and I. Pekova, Chem. Zvesti, 36 (1982) 169.
M. Ebert, Z. Mička and I. Pekova, Collect. Czech. Chem. Commun., 47 (1982) 2069.
G. G. Gospodinov, L. M. Sukhova and K. I. Petrov, Zh. Neorg. Khim., 33 (1988) 1970 (in Russian).
P. P. Pradyumnan and M. A. Ittyachen, J. Therm. Anal. Cal., 61 (2000) 243.
L. I. Martynenko and V. I. Spitzin, Metodicheskie aspekty kursa neorganicheskoi khimii, Moscow University, Moscow, 1983, (in Russian).
A. A. Frost and R. G. Pearson, Kinetics and Mechanism of Chemical Reactions, John Wiley and Sons Inc., New York, 1961.
L. K. Singh and S. Mitra, Thermochim. Acta, 138 (1989) 285.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vlaev, L.T., Georgieva, V.G. & Genieva, S.D. Products and kinetics of non-isothermal decomposition of vanadium(IV) oxide compounds. J Therm Anal Calorim 88, 805–812 (2007). https://doi.org/10.1007/s10973-005-7149-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-005-7149-y