Abstract
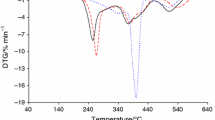
Thermal decomposition kinetics of calix[6]arene (C6) and calix[8]arene (C8) were studied by Thermogravimetry analysis (TG) and Differential thermal analysis (DTA). TG was done under static air atmosphere with dynamic heating rates of 1.0, 2.5, 5.0, and 10.0 K min−1. Model-free methods such as Friedman and Ozawa–Flynn–Wall were used to evaluate the kinetic parameters such as activation energy (E a) and pre-exponential factors (ln A). Model-fitting method such as linear regression was used for the evaluation of optimum kinetic triplets. The kinetic parameters obtained are comparable with both the model-free and model-fitting methods. Within the tested models, the thermal decomposition of C6 and C8 are best described by a three dimensional Jander’s type diffusion. The antioxidant efficiency of C6 and C8 was tested for the decomposition of polypropylene (PP).









Similar content being viewed by others
References
Gutsche CD. Calixarenes. Monographs in supramolecular chemistry. London: Royal Society of Chemistry; 1989.
Asfari Z, Bohmer V, Harrowfield J, Vicens J, Saadioui M. Calixarenes 2001. Netherlands: Kluwer Academic Publishers; 2001.
Radhakrishnan Nair MN, Thomas GV, Gopinathan Nair MR. Thermogravimetric analysis of PVC/ELNR blends. Polym Degrad Stab. 2007;92:189–96.
Yao F, Wu Q, Lei Y, Guo W, Xu Y. Thermal decomposition kinetics of fibers: activation energy with dynamic thermo gravimetric analysis. Polym Degrad Stab. 2008;93:90–8.
Vyazovkin S. Thermal analysis. Anal Chem. 2008;80:4301–16.
Lazzarotto M, Nachtigall FF, Schnitzler E, Castellano EE. Thermo gravimetric analysis of supramolecular complexes of p-tert-butylcalix[6]arene and ammonium cations: crystal structure of diethylammonium complex. Thermochim Acta. 2005;429:111–7.
Schatz J, Schildbach F, Lentz A, Rastatter S. Thermal gravimetry, mass spectrometry and solid-state 13C NMR spectroscopy-simple and efficient methods to characterize the inclusion behavior of p-tert-butylcalix[n]arenes. J Chem Soc Perkin Trans 2. 1998;(1):75–7.
Pastor SD, Odorisio P. Acylated calixarene stabilizers. US Patent 4,617,336. 1986.
Seiffarth K, Schulz M, Gormar G, Bachmann J. Calix[n]arenes-new light stabilizers for polyolefins. Polym Degrad Stab. 1989;24:73–80.
Feng W, Yuan LH, Zheng SY, Huang GL, Qiao JL, Zhou Y. The effect of p-tert-butylcalix[n]arene on γ-radiation degradation of polypropylene. Radiat Phys Chem. 2000;57:425–9.
Zaharescu T, Jipa S, Setnescu R, Santos C, Gigante B, Mihalcea I, Podina C, Gorghiu LM. Thermal stability of additivated isotactic polypropylene. Polym Bull. 2002;49:289–96.
Jipa S, Zaharescu T, Setnescu R, Setnescu T, Dumitru M, Gorghiu LM, Mihalcea I, Bumbac M. Effect of calixarenes on thermal stability of polyethylenes. Polym Degrad Stab. 2003;80:203–8.
Pospisil J. Mechanistic action of phenolic antioxidants in polymers—a review. Polym Degrad Stab. 1988;20:181–202.
Gutsche CD, Dhawan B, No KH, Muthukrishnan R. Calixarenes. 4. The synthesis, characterization and properties of the calixarenes from p-tert-butylphenol. J Am Chem Soc. 1981;103:3782–92.
Gutsche CD, Lin LG. Calixarenes 12: the synthesis of functionalized calixarenes. Tetrahedron. 1986;42(6):1633–40.
Galwey AK, Brown ME. Handbook of thermal analysis and calorimetry, vol. 1. Amsterdam: Elsevier; 1998.
Friedman H. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J Polym Sci. 1963;6(1):183–95.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881–6.
Flynn JH, Wall LA. General treatment of the thermogravimetry of polymers. J Res Natl Bur Stand. 1996;70A:487–523.
Vyazovkin S, Wight CA. Model-free and model-fitting approaches to kinetic analysis of isothermal and non-isothermal data. Thermochim Acta. 1999;340–341:53–68.
Swain SN, Rao KK, Nayak PL. Biodegradable polymers part II. Thermal degradation of biodegradable plastics cross-linked from formaldehyde-soy protein concentrate. J Therm Anal Calorim. 2005;79:33–8.
Turmanova SC, Genieva SD, Dimitrova AS, Vlaev LT. Non-isothermal degradation kinetics of filled with rise husk ash polypropene composites. Exp Polym Lett. 2008;2(2):133–46.
Chen F, Sorensen OT, Meng G, Peng D. Thermal decomposition of BaC2O4.5H2O Studied by stepwise isothermal analysis and non-isothermal thermogravimetry. J Therm Anal Calorim. 1998;53:397–410.
Vyazovkin S. A unified approach to kinetic processing of non-isothermal data. Int J Chem Kinet. 1996;28:95–101.
Pielichowski K, Njuguna J. Thermal degradation of polymeric materials. UK: Rapra Technology Limited; 2005.
Deligoz H, Ozen O, Cilgi GK, Cetisli H. A study on the thermal behaviours of parent calix[4]arenes and some azocalix[4]arene derivatives. Thermochim Acta. 2005;426:33–8.
Zhu L, Chen J, Xu L, Lian X, Xu K, Chen M. Synthesis of 3,5-ditert-butyl-4-hydroxybenzoates and their thermal antioxidation behavior for polypropylene. Polym Degrad Stab. 2009;94:1906–13.
Maskos Z, Khachatryan L, Dellinger B. Formation of the persistent primary radicals from the pyrolysis of tobacco. Energy Fuels. 2008;22:1027–33.
Tanaka A, Yashiro H, Ishigaki A, Murai H. Time-resolved ESR study on complex radical pairs formed in the photolysis of methylene blue included in water-soluble sulfonated calixarenes. Appl Magn Reson. 2010;37:581–93.
Wang Q, Li Y, Wu GS. ESR study of calix[4]arene by spin-trapping method. Appl Magn Reson. 2000;18:419–24.
Qin Z, Xin Z, Jian-Bing Z, Jun T, Zhao-Tian F, Wan-Fu S. Radiation effect of Apocynum fiber. Nucl Sci Tech. 2006;17:38–42.
Hedrick SA, Chuang SSC. Temperature programmed decomposition of polypropylene: in situ FTIR coupled with mass spectroscopy study. Thermochim Acta. 1998;315:159–68.
Yang W, Manek R, Kolling WM, Brits M, Liebenberg W, De Villiers MM. Physicochemical characterization of hydrated 4-sulphonato-calix[n]arenas: thermal, structural and sorption properties. Supramol Chem. 2005;17:485–96.
Aboulkas A, Harfi KE, Bouadili AE, Chanaa MB, Mokhlisse A. Pyrolysis kinetics of polypropylene morocco oil shale and their mixture. J Thermal Anal Calorim. 2007;89:203–9.
Acknowledgements
The authors K. Chennakesavulu and M. Raviathul Basariya Senior Research Fellows are grateful to Council of Scientific and Industrial Research (CSIR), New Delhi (India) for financial support. The authors are grateful to SAIF-NMR Facility, Indian Institute of Technology, Madras (India), NMR Centre-Indian Institute of Science, Bangalore (India) for providing the necessary spectral and analytical data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chennakesavulu, K., Raviathul Basariya, M., Bhaskar Raju, G. et al. Study on thermal decomposition of calix[6]arene and calix[8]arene. J Therm Anal Calorim 103, 853–862 (2011). https://doi.org/10.1007/s10973-010-1065-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-010-1065-5