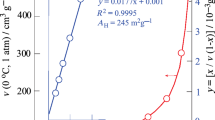
Abstract
Water vapor adsorption on silica gel was investigated using Tian–Calvet-type microcalorimetry. Differential heat of adsorption data was obtained. The setup of microcalorimetry was used volumetric system to determine adsorption isotherms of water vapor–silica gel. The Langmuir model was used in the interpretation of the adsorption data. The Clausius–Clapeyron diagram was also given. Effective mass diffusivity of water vapor in the silica gel particle as a function of temperature was also determined. The silica gel, which was degassed under vacuum at 10−7 mbar and 120 °C for 18 h, was found to adsorb 0.6, 0.98, 1.1, 1.4, 2, 3.5, 11, 13, and 14 wt% water vapor at 120, 110, 100, 90, 75, 60, 40, 35, and 30 °C, respectively. The diffusivities of water vapor inside the silica gel for short- and long-range periods were described using kinetics data as a function of temperature in the Arrhenius form.







Similar content being viewed by others
Abbreviations
- b :
-
Langmuir constant
- C ∞ :
-
Equilibrium concentration, kg m−3
- D eff :
-
Effective diffusivity, m2 s−1
- D 0 :
-
Reference diffusivity, m2 s−1
- ∆H st :
-
Heat of vaporization, kJ kg −1w
- ΔH a :
-
Integral heat of adsorption, kJ kg−1
- \( \Updelta \overline{h}^{\text{a}} \) :
-
Differential heat of adsorption, kJ mol−1
- E :
-
Diffusion activation energy, J mol−1
- n :
-
Eigen value
- n a :
-
Amount of adsorbate mol kg−1
- P :
-
Pressure, kPa
- r p :
-
Radius of adsorbent granule, m
- R :
-
Ideal gas constant, J mol−1 K−1
- t :
-
Time, s
- T :
-
Temperature, K
- W :
-
Average adsorbate concentration, kgw kg −1S
- W m :
-
Monolayer adsorption coverage, kgw kg −1S
- \( \mathop {W_{\text{t}} }\limits^{ - } \) :
-
Average adsorption coverage at time t, kgw kg −1S
- W ∞ :
-
Adsorbate concentration in equilibrium, kgW kg −1s
References
Demir H, Mobedi M, Ülkü S. A review on adsorption heat pump: problems and solutions. Renew Sustain Energy Rev. 2008;12:2381–403.
Ülkü S. Adsorption heat pumps. J Heat Recovery Sys. 1986;6:277–84.
Ülkü S, Mobedi M. Adsorption in energy storage. Proc NATO Adv Stud Inst Energy Storage Syst Ser E Appl Sci. 1989;167:487–507.
Ülkü S. In: Yüncü H, Paykoç E, Yener Y, editors. Solar adsorption heat pumps. In: Solar energy utilization: fundamentals and applications. Netherlands: Martinus Nijkoff Publishers; 1987.
Schnabel L, Henning MH. Experimental and simulation study on the kinetics of water vapour adsorption on different kinds of adsorptive material matrices. In: International sorption heat pump conference, Denver, USA, 2005.
Karger J, Ruthven DM. Diffusion in zeolites and other microporous solids. New York: Wiley-Interscience Pubs; 1992.
Ruthven DM. Principles of adsorption and adsorption processes. New York: Wiley-Interscience Pubs; 1984.
Aristov YI, Tokarev MM, Freni A, Glaznev SI, Restuccia G. Kinetics of water adsorption on silica Fuji Davison RD. Microporous Mesoporous Mater. 2006;96:65–71.
Wang X, Zimmermann W, Ng CK, Chakraboty A, Keller UJ. Investigation on the isotherm of silica gel-water systems TG and volumetric methods. J Therm Anal Calorim. 2004;76:659–69.
Wang J, Cheng D, Zeng N, Xia H, Fu Y, Yan D, Zhao Y, Xiao X. Application of microcalorimetry and principal component analysis: antibacterial evaluation of Benzoinum and Styrax on Staphylococcus aureus growth. J Therm Anal Calorim. 2010;102:137–42.
Bulanek R, Frolich K, Frydova E, Cicmanec P. Study of adsorption sites heterogeneity in zeolites by means of coupled microcalorimetry with volumetry. J Therm Anal Calorim. doi:10.1007/s10973-010-1108-y.
Schabes IF, Sigstad EE. Monitoring soybean seed germination by calorimetry. J Therm Anal Calorim. doi:10.1007/s10973-010-1036-x.
Moise JC, Bellat JP, Methiever A. Adsorption of water vapor on X and Y zeolites exchanged with barium. Microporous Mesoporous Mater. 2001;43:91–101.
Ozkan FC, Ulku S. The effect of HCl treatment on water vapor adsorption characteristics of clinoptilolite rich natural zeolite. Microporous Mesoporous Mater. 2005;77:47–53.
Ulku S. Natural zeolites in energy storage and heat pumps. Surf Sci Catal. 1986;28:1047–54.
Janchen J, Ackermann D, Weiler E, Stach H, Brösicke W. Calorimetric investigation on zeolites, AlPO4’s and CaCl2 impregnated attapulgite for thermo-chemical storage of heat. Thermochim Acta. 2005;434:37–41.
Myers AL. Thermodynamics of adsorption in porous materials. AIChE J. 2002;48:145–60.
Ben Amar N, Sun ML, Meunier F. Numerical analysis of adsorptive temperature wave regenerative heat pump. Appl Therm Eng. 1996;16:405–18.
Acknowledgements
Authors would like to thank to State Planning Organization of Turkey for their great financial supports to this project 2003K120690 (DPT-6).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Demir, H., Mobedi, M. & Ülkü, S. Microcalorimetric investigation of water vapor adsorption on silica gel. J Therm Anal Calorim 105, 375–382 (2011). https://doi.org/10.1007/s10973-011-1395-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-1395-y