Abstract
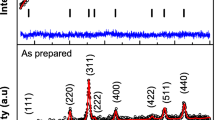
Magnetic nanoparticles of cobalt ferrite have been synthesized by citrate precursor method. TG-DSC studies have been made to get the idea of the optimum temperature of annealing that could lead to the formation of nanoparticles. Annealing the citrate precursor was done at 450, 650, and 973 °C. The X-ray diffraction (XRD) studies and the scanning electron microscopy (SEM) have been used for characterization. The data from vibrating sample magnetometer and photoluminescence spectrometer (PL) have been analyzed for exploring their applications. Using the Scherrer formula, the crystallite size was found to be 25, 32, and 43 nm, respectively, using the three temperatures. The particle size increased with annealing temperature. Rietveld refinements on the X-ray (XRD) data were done on the cobalt ferrite nanoparticles (monoclinic cells) obtained on annealing at 650 °C, selecting the space group P2/M. The values of coercivity (1574.4 G) and retentivity (18.705 emu g−1) were found out in the sample annealed at 650 °C while magnetization (39.032 emu g−1) was also found in the sample annealed at 973 °C. The photoluminescence (PL) property of these samples were studied using 225, 330, and 350 nm excitation wavelength radiation source. The PL intensity was found to be increasing with the particle size.







Similar content being viewed by others
References
Zi Z, Sun Y, Zhu X, Yang Z, Dai J, Song W. Synthesis and magnetic properties of CoFe2O4 ferrite nanoparticles. J Magn Magn Mater. 2009;321:1251–5.
Grigorova M, Blythe HJ, Blaskov V, Rusanov V, Petkov V, Masheva V, Nihtianova D, Martinez LM, Munoz JS, Mikhov M. Magnetic properties and Mössbauer spectra of nanosized CoFe2O4 powders. J Magn Magn Mater. 1998;183:163–72.
Shenker H. Magnetic anisotropy of cobalt ferrite (Co1.01Fe2.00O3.62) and nickel cobalt ferrite (Ni0.72Fe0.20Co0.08Fe2O4). Phys Rev. 1957;107:1246–9.
West AR. Basic solid state chemistry. Delhi: Wiley; 1998. p. 356.
Salunkhe AB, Khot VM, Phadatare MR, Pawar SH. Combustion synthesis of cobalt ferrite nanoparticles—influence of fuel to oxidizer ratio. J Alloy Compd. 2012;514:91–6.
Maaz K, Mumtaz A, Hasnain SK, Ceylan A. Synthesis and magnetic properties of cobalt ferrite (CoFe2O4) nanoparticles prepared by wet chemical route. J Magn Magn Mater. 2007;308:289–95.
Naidek KP, Bianconi F, Rizuti da Rocha TC, Zanchet D, Bonacin JA, Novak MA, Vaz MGF, Winnischofer H. Structure and morphology of spinel MFe2O4 (M = Fe, Co., Ni) nanoparticles chemically synthesized from heterometallic complexes. J Colloid Interface Sci. 2011;358:39–46.
Cedeno-Mattei Y, Perales-Perez O. Synthesis of high-coercivity cobalt ferrite nanocrystals. Microelectron J. 2009;40:673–6.
Fannin PC, Marin CN, Malaescu I, Stefu N, Vlazan P, Novaconi S, Sfirloaga P, Popescu S, Couper C. Microwave absorbent properties of nanosized cobalt ferrite powders prepared by coprecipitation and subjected to different temperature treatments. Mater Des. 2011;32:1600–4.
Zhang Y, Yang Z, Yin D, Liu Y, Fei C, Xiong R, Shi J, Yan G. Composition and magnetic properties of cobalt ferrite nano-particles prepared by the co-precipitation method. J Magn Magn Mater. 2010;322:3470–5.
Sajjia M, Oubaha M, Prescott T, Olabi AG. Development of cobalt ferrite powder preparation employing the sol–gel technique and its structural characterization. J Alloy Compd. 2010;506:400–6.
Verma RK, Hill JO, Niinisto L, Mojumdar SC, Kumar DD. A curriculum framework for education in thermal analysis. J Mater Educ. 2012;34:133–50.
Verma RK, Verma L, Chandra M. Thermoanalytical studies on the non-isothermal dehydration and decomposition of dl-lactates of a series of transition metals. Indian J Chem. 2003;42A:2982–7.
Bhattacharjee NC, Kumar M, Kumar S, Verma RK. Kinetic and mechanistic studies on non-isothermal decomposition of potassium dioxalatocuprate(II) dihydrate. J Indian Chem Soc. 1998;75(5):317–8.
Verma RK, Verma L, Chandra M, Bhushan A. Non-isothermal dehydration and decomposition of dl-lactates of transition metals and alkaline earth metals: a comparative study. J Therm Anal Calorim. 2005;80:351–4.
Kumar M, Verma RK, Verma L, Bhattacharjee NC, Kumar S, Verma BP. Thermal decomposition of potassium trioxalato chromate(III) trihydrate: a kinetic and mechanistic study. Asian J Chem. 1996;8(3):543–6.
Agrawal HL, Mishra A, Ambasta RK, Verma L, Verma RK, Verma BP. Kinetic parameters of thermolysis of complexes of rhodium(III), palladium(II) and platinum(II) with substituted morpholines from their non-isothermal thermogravimetric data. Asian J Chem. 1994;6:130–4.
Verma BP, Verma RK, Chandra M, Pandey S, Mallick AK, Verma L. A study of non-isothermal decomposition of calcium dl-lactate pentahydrate. Asian J Chem. 1994;6:606–12.
Verma RK, Verma L, Ranjan M, Verma BP, Mojumdar SC. Thermal analysis of 2-oxocyclopentanedithiocarboxylato complexes of iron(III), copper(II) and zinc(II) containing pyridine or morpholine as the second ligand. J Therm Anal Calorim. 2008;94:27–31.
Verma RK, Verma L, Bhushan A, Verma BP. Thermal decomposition of complexes of cadmium(II) and mercury(II) with triphenylphosphanes. J Therm Anal Calorim. 2007;90:725–9.
Brown ME, Gallagher PK. Introduction to recent advances, techniques and applications of thermal analysis and Calorimetry. In Brown ME, Gallagher PK, Editors. Hand book of thermal analysis and calorimetry. Elsevier: New York; 2008. pp. 1–12.
Verma RK, Verma L, Chandra M, Verma BP. Kinetic parameters of thermal dehydration and decomposition from thermoanalytical curves of zinc dl-lactate. J Indian Chem Soc. 1998;75:162–4.
Singh RK, Yadav A, Narayan A, Singh AK, Verma L, Verma RK. Thermal, structural and magnetic studies on chromite spinel, synthesized by citrate precursor method and annealed at temperature 450 °C and 650 °C. J Therm Anal Calorim. 2012;107:197–204.
Singh RK, Yadav A, Narayan A, Chandra M, Verma RK. Thermal, XRD and magnetization studies on ZnAl2O4 and NiAl2O4 spinels, synthesized by citrate precursor method and annealed at temperature 450°C and 650°C. J Therm Anal Calorim. 2012;107:205–10.
Goldstein AN (ed.), Handbook of nanophase materials. New York: Marcel Dekker; 1997. p. 1.
Panda RN, et al. Magnetic properties of nano-crystalline Gd or Pr substituted CoFe2O4 synthesized by the citrate precursor technique. J Magn Magn Mater. 2003;257:79–86.
Sajjia M, Benyounis KY, Olabi AG. The simulation and optimization of heat treatment of cobalt ferrite nanoparticles prepared by sol–gel technique. Powder Technol. 2012;222:143–51.
Cullity BD. Elements of X-ray diffraction. New York: Wiley; 1978. p. 101.
Roisnel J, Rodrıguez-Carvajal J, WinPLOTR; Laboratoire Leon Brillouin (CEA-CNRS) Centre d’Etudes de Saclay: Gif sur Yvette Cedex, France, 2000.
Rodriguez-Carvajal J. FullProf 2000: A Rietveld Refinement and Pattern Matching Analysis Program, (Version: April 2008), Laboratoire Léon Brillouin (CEA-CNRS), France.
Williamson GK, Hall WH. X-ray line broadening from filed aluminium and wolfram. Acta Metal. 1953;1:22.
Bhowmik RN. Lattice expansion and magnetic order in spinel oxide. Kolkata: Proceedings of National Conference on Nanoscience & Nanotechnology; 2009.
Chinnasamy CN, Jeyadevan B, Perales-Perez O, Shinoda K, Tohji K, Kasuya A. Growth dominant co-precipitation process to achieve high coercivity at room temperature in CoFe2O4 nanoparticles. IEEE Trans Magn. 2002;38(5):2640–2.
Bahadur D. Current trends in applications of magnetic ceramic materials. Bull Mater Sci. 1992;15(5):431–9.
Bandyopadhay AK. Nanomaterials. New. Delhi: New age international; 2007. pp. 254–267.
Xi YY, Cheung TLY, Dickon HLN. Synthesis of ternary Zn x Cd1−xS nanowires by thermal evaporation and the study of their photoluminescence. Mater Lett. 2008;62:128–32.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, R.K., Narayan, A., Prasad, K. et al. Thermal, structural, magnetic and photoluminescence studies on cobalt ferrite nanoparticles obtained by citrate precursor method. J Therm Anal Calorim 110, 573–580 (2012). https://doi.org/10.1007/s10973-012-2728-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2728-1