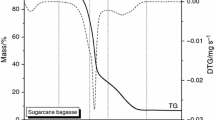
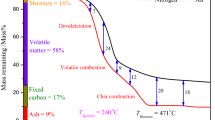
Abstract
Thermogravimetric analysis was used to study and compare the combustion of different blends of corn bioresidues with sunflower, rape and algae bioresidues. Non-isothermal thermogravimetric data were used to obtain the combustion kinetics of these bioresidues. This paper reports on the application of the Vyazovkin and Ozawa–Flynn–Wall isoconversional methods for the evaluation of kinetic parameters (energy activation, pre-exponential factor and order of reaction) for the combustion of the biomasses studied. Differences were found in the TG curves in accordance with the proximate analysis results for the cellulose, hemicellulose and lignin content of biomasses. The activation energy obtained from combustion (E ~ 151.6 kJ mol−1) was lower than that from the blends (similar values were obtained for corn–sunflower, E ~ 160.5 kJ mol−1 and corn–rape, E ~ 156.9 kJ mol−1) whereas the activation energy obtained from the microalgae was higher (E ~ 171.5 kJ mol−1). Both the Vyazovkin and Ozawa–Flynn–Wall methods yielded similar results.









Similar content being viewed by others
References
Khan AA, de Jong W, Jansens PJ, Spliethoff H. Biomass combustion in fluidized bed boilers: potential problems and remedies. Fuel Process Technol. 2009;90:21–50.
Berndes G, Hoogwijk M, Van den Broek R. The contribution of biomass in the future global energy supply: a review of 17 studies. Biomass Bioenergy. 2003;25:1–28.
Mckendry P. Energy production from biomass (part. 1): overview of biomass. Bioresour Technol. 2002;83:37–46.
Sanchez-Silva L, Lopez-Gonzalez D, Villaseñor J, Sanchez P, Valverde JL. Thermogravimetric-mass spectrometric analysis of lignocellulosic and marine biomass pyrolysis. Bioresour Technol. 2012;109:163–72.
Demirbas A. Potential applications of renewable energy sources, biomass combustion problems in boiler power systems and combustion related environmental issues. Prog Energy Combust Sci. 2005;31:171–92.
Qiu G. Testing of flue gas emissions of a biomass pellet boiler and abatement of particle emissions. Renew Energy. 2012;50:94–102.
Fangxian L, Shizong L, Youzhi C. Thermal analysis study of the effect of coal-burning additives on the combustion of coals. J Therm Anal Calorim. 2009;95:633–8.
Xiao H, Ma X, Lai Z. Isoconversional kinetic analysis of co-combustion of sewage sludge with straw and coal. Appl Energy. 2009;86:1741–5.
Gil MV, Riaza J, Álvarez L, Pevida C, Pis JJ, Rubiera F. A study of oxy-coal combustion with steam addition and biomass blending by thermogravimetric analysis. J Therm Anal Calorim. 2012;109:49–55.
Gil MV, Casal D, Pevida C, Pis JJ, Rubiera F. Thermal behaviour and kinetics of coal/biomass blends during co-combustion. Bioresour Technol. 2010;101:5601–8.
Yorulmaz SY, Atimtay AT. Investigation of combustion kinetics of treated and untreated waste wood samples with thermogravimetric analysis. Fuel Process Technol. 2009;90(7–8):939–46.
Sanchez ME, Otero M, Gomez X, Moran A. Thermogravimetric kinetic analysis of the combustion of biowastes. Renew Energy. 2009;34:1622–7.
Pis JJ, de la Puente G, Fuente E, Morán A, Rubiera F. A study of the self-heating of fresh and oxidized coals by differential thermal analysis. Thermochim Acta. 1996;279:93–101.
Haykiri-Açma H. Combustion characteristics of different biomass materials. Energy Convers Manag. 2003;44:155–62.
Vyazovkin S, Lisnikovick AI. Transformation of “degree of conversion against temperature” into “degree of conversion against time” kinetic data. Russ J Phys Chem. 1988;62:1525–7.
Vyazovkin S, Wight CA. Isothermal and non-isothermal kinetics of thermally simulated reactions of solids. Int Rev Phys Chem. 1998;17:407–33.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881–6.
Flynn JH, Wall LA. A quick, direct method for the determination of activation energy from thermogravimetric data. Polym Lett. 1966;4:323–8.
Tai Tan F, Wian Ma X, Feng C. Investigation on combustion of fire retardant board under different N2–O2 mixtures gas atmospheres by using thermogravimetric analysis. Constr Build Mater. 2011;25:2076–84.
Varol M, Atimtay AT, Bay B, Olgun H. Investigation of co-combustion characteristics of low quality lignite coals and biomass with thermogravimetric analysis. Thermochim Acta. 2010;510:195–201.
Van Soest PJ, Robertson JB, Lewis BA. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci. 1991;74:3583–97.
Gentziz T, Chambers A. Physical structure changes of Canadian coals during combustion. Energy Sour. 1995;17:131–49.
Ozawa T. Kinetic analysis of derivative curves in thermal analysis. J Therm Anal. 1970;2:301–24.
Kneller WA. Physicochemical characterization of coal and coal reactivity: a review. Thermochim Acta. 1986;108:357–88.
Ma B, Li X, Xu L, Wang K, Wang X. Investigation on catalyzed combustion of high ash coal by thermogravimetric analysis. Thermochim Acta. 2006;445:19–22.
Nie Q-H, Sun S-Z, Li Z-Q. Thermogravimetric study on the combustion characteristics of brown coal blends. J Combust Sci Technol. 2001;7(1):72–6.
Li X-G, Ma B-G. Thermogravimetric analysis of the co-combustion of the blends with high ash coal and waste tyres. Thermochim Acta. 2006;441(1):79–83.
Xie J-L, He F. Catalyzed combustion study of anthracite in cement kiln. J Chin Ceram Soc. 1998;26(6):792–5.
Otero M, Sánchez ME, Gómez X, Morán A. Thermogravimetric analysis of biowastes during combustion. Waste Manag. 2010;30:1183–7.
Sima-Ella E, Mays TJ. Analysis of the oxidation reactivity of carbonaceous materials using thermogravimetric analysis. J Therm Anal Calorim. 2005;80:109–13.
Vyazovkin S. Model-free kinetics. Staying free of multiplying entities without necessity. J Therm Anal Calorim. 2006;83:45–51.
Khawam A, Flanagan DR. Role of isoconversional methods in varying activation energies of solid-state kinetics: I. Isothermal kinetic studies. Thermochim Acta. 2005;429:93–102.
Vyazovkin S, Dollimore D. Linear and nonlinear procedures in isoconversional computations of the activation energy of nonisothermal reactions in solids. J Chem Inf Comput Sci. 1996;36:42–5.
Moreno RMB, Medeiros ES, Ferreira FC, Alves N, Gonçalves PS, Mattoso LHC. Thermogravimetric studies of decomposition kinetics of six different IAC Hevea rubber clones using Flynn–Wall–Ozawa approach. Plast Rubber Compos. 2006;35:15–21.
Ramajo-Escalera B, Espina A, García JR, Sosa-Arnao JH, Nebra SA. Model-free kinetics applied to sugarcane bagasse combustion. Thermochim Acta. 2006;448:111–6.
Silva AR, Crespi MS, Ribeiro CA, Oliveira SC, Silva MRS. Kinetic of thermal decomposition of residues from different kinds of composting. J Therm Anal Calorim. 2004;75:401–9.
Doyle CD. Estimating isothermal life from thermogravimetric data. J Appl Polym Sci. 1962;6:639–42.
Tang Y, Ma X, Lai Z. Thermogravimetric analysis of combustion of microalgae and microalgae blended with waste in N2/O2 and CO2/O2 atmospheres. Bioresour Technol. 2011;102:1879–85.
Haykiri-Açma H, Ersoy-Meriçboyu A, Küçükbayrak S. Effect of demineralization on the reactivity of lignites. Thermochim Acta. 2000;362:131–5.
Küçükbayrak S, Haykiri-Açma H, Ersoy-Meriçboyu A. Effect of lignite properties on reactivity of lignite. Energy Convers Manag. 2001;42:613–26.
Haykiri-Açma H, Ersoy-Meriçboyu A, Küçükbayrak S. Effect of mineral matter on the reactivity of lignite chars. Energy Convers Manag. 2001;42:11–20.
Aboyade A, Hugo TJ, Carrier M, Meyer EL, Stahl R, Knoetze JH, Görgens JH. Non-isothermal kinetic analysis of the devolatilization of corn cobs and sugar cane bagasse in an inert atmosphere. Thermochim Acta. 2011;517:81–9.
Zhao H, Yan H, Dong S, Zhang Y, Sun B, Zhang C, Ai Y, Chen B, Liu Q, Sui T, Qin S. Thermogravimetry study of the pyrolytic characteristics and kinetics of macro-algae Macrocystis pyrifera residue. J Therm Anal Calorim. 2011. doi:10.1007/s10973-011-2102-8.
Ernesto VART, Ribeiro CA, Hojo O, Fertonani FL, Crespi MS. Thermal characterization of lignocellulosic residue from different sugarcanes. J Therm Anal Calorim. 2009;97:653–6.
Wang XB, Si JP, Tan HZ, Niu YQ, Xu C, Xu TM. Kinetics investigation on the combustion of waste capsicum stalks in Western China using thermogravimetric analysis. J Therm Anal Calorim. 2012;109:403–12.
Acknowledgements
This research was supported by the Natural Resources Institute of University of León, which provided human and material assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
López, R., Fernández, C., Gómez, X. et al. Thermogravimetric analysis of lignocellulosic and microalgae biomasses and their blends during combustion. J Therm Anal Calorim 114, 295–305 (2013). https://doi.org/10.1007/s10973-012-2843-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2843-z